Refractory angina (RA) is defined as chronic angina-type chest pain (duration ≥ 3 months) associated with reversible ischaemia that persists despite optimal medical, interventional and surgical management.1
The clinical burden of RA is growing due to an ageing population and improved survival from coronary artery disease (CAD). Estimates suggest that in the US between 600,000 and 1.8 million patients suffer from RA, with 75,000 new patients diagnosed each year. In Canada, approximately half a million patients live with RA, while in Europe, 30,000–50,000 new cases are diagnosed per year.2,3 Data on the epidemiology of RA in the UK are lacking and further work to define the disease burden is needed.
The successful management of RA is often extremely challenging. Povsic et al. showed that patients with RA were more frequently hospitalised, often undergoing angiographic investigation without revascularisation and consequently incurring US$10,108 greater healthcare costs per patient over a 3-year period compared to a matched control group.4 Coupled with evidence that RA is not associated with worse long-term mortality, novel treatment approaches targeted at improving symptoms and quality of life in this challenging patient population are needed.5
Patients with RA may be more appropriately considered as having a chronic chest pain syndrome with both physical and psychological components that may require the implementation of pharmacological and psychological approaches as well as interventional strategies. 6–9 In this review, we focus on advances in the interventional management of RA, specifically coronary sinus reducer (CSR) implantation, external enhanced counterpulsation (EECP), extracorporeal shockwave therapy (ECSWT) and cell therapy.
Coronary Sinus Reducer
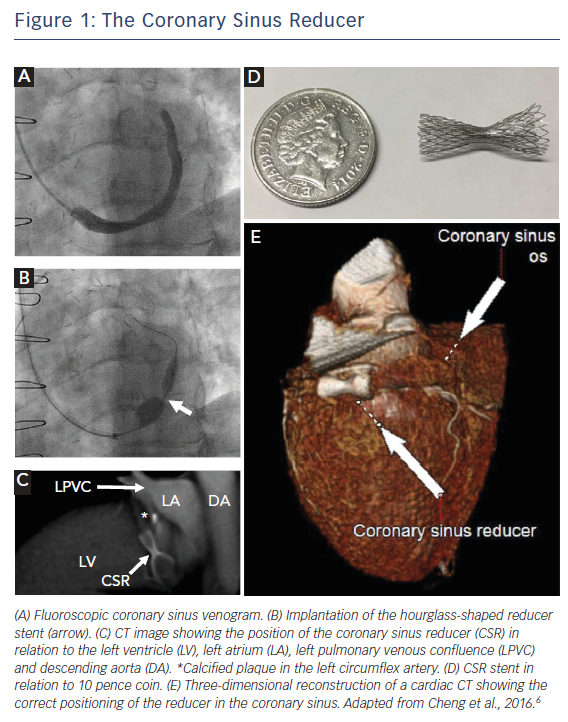
The CSR is a balloon catheter-mounted hourglass-shaped stent that has been shown to improve symptoms and quality of life in patients with RA (Figure 1). Stenting of the coronary sinus to produce a stenosis and increase venous backpressure has gained traction recently as a means of improving perfusion by diverting blood to ischaemic myocardium. It follows from studies originating from 1955 by Beck and colleagues at the Cleveland Clinic whereby experimental narrowing of the coronary sinus was able to reduce myocardial infarct size by increasing retrograde perfusion.10 Early translation into 185 patients with severe CAD who underwent surgical narrowing of the coronary sinus (through an open chest approach to produce a 60–70 % narrowing and achieve a 3 mm residual lumen diameter of the coronary sinus) demonstrated significant improvements in anginal symptoms, medication use and mortality.11,12 However, interest in this concept waned as interventions to improve arterial inflow, i.e. coronary bypass grafting and percutaneous coronary interventions, gained prominence in the latter half of the 20th century. More recent preclinical studies have shown that occlusion of the coronary sinus can preserve the endocardial-to-epicardial perfusion ratio, improving subendocardial perfusion by redistributing blood flow from non-ischaemic to ischaemic areas.11 To achieve this via a minimally invasive percutaneous approach, the CSR was developed as a catheter-mounted balloon-inflatable stent that can be implanted via a trans-jugular approach.
In normal physiology, exercise-induced sympathetic-mediated vasoconstriction of epicardial arteries promotes blood flow to the subendocardium (subendocardial:subepicardial perfusion ratio: ~1.2).13 In the presence of epicardial CAD this is dysfunctional and, together with regional wall motion abnormalities with consequent raised left ventricular end-diastolic pressure and compression of the subendocardial capillaries, perfusion falls (perfusion ratio: 0.8). By raising venous backpressure through coronary sinus occlusion, venules are dilated and resistance to subendocardial perfusion is reduced, with collaterals recruited between the subepicardium and subendocardium.
First-in-man studies by Banai et al. demonstrated the safety of device implantation and reported improvements in angina score in 12 out of 14 patients. Canadian Cardiovascular Society (CCS) class was significantly improved (from 3.07 to 1.64, p<0.0001), together with stress-induced ST-segment depression and myocardial ischaemia on dobutamine echocardiography and single-photon emission computed tomography (SPECT).14 Endothelialisation of the CSR takes about 6 weeks. In our centre, patients are counselled that they should not gauge success immediately after implantation but should await a more accurate assessment of treatment response at several months follow-up. Anecdotal evidence exists as to immediate symptomatic benefit after implantation. Possible reasons for this include a significant placebo effect and device implantation over a valve in the coronary sinus leading to early endothelialisation. Questions have been raised as to the feasibility of using the coronary sinus for cardiac resynchronisation therapy.15 CSR implantation is unlikely to improve symptoms of exertional dyspnoea in severe left ventricular systolic dysfunction and although the stent can be further dilated for cardiac resynchronisation therapy, CSR implantation in these patients is not advisable.12 Furthermore, given that optimal CSR implantation is approximately 2 cm distal to the ostium of the coronary sinus, where the middle cardiac vein drains the right coronary territory, any backpressure generated is unlikely to affect this territory. CSR implantation is only suitable for left-sided ischaemia.
The largest study to date was the Phase II randomised, blinded, sham-controlled Coronary Sinus Reducer for Treatment of Refractory Angina (COSIRA) trial.16 Of 104 patients with CCS III–IV RA, 35 % of treated patients compared to 15 % of controls met the primary endpoint a reduction of ≥2 CCS classes at 6-month follow-up (p=0.02). Encouragingly, improvements in ≥1 CCS were significant (71 % versus 42 %, p=0.003) as were improvements in quality of life (p=0.03) as assessed with the Seattle Angina Questionnaire (SAQ). Interestingly, no changes were seen in exercise time or mean change in wall-motion index on dobutamine echocardiography, although it should be noted that the study was not powered to meet these endpoints.
The CSR may also improve psychosocial outcomes such as anxiety and depression.17 Moreover, high rates of procedural success have been demonstrated (91 and 96 %), with unsuitable coronary sinus anatomy and the presence of venous valves accounting for the small proportion of failures.16,18 CSR implantation also has low rates of adverse events – the COSIRA trial reported only one case of periprocedural serious adverse event in the CSR cohort (n=50) and the rates of other serious adverse events were similar to the sham control group. There was no evidence of device migration in patients followed-up with CT angiography (n=36). Given the success of the COSIRA trial, which predominantly included centres in Canada and Europe, a Phase III multicentre, randomised, double-blind sham-controlled trial (COSIRA II) is planned in the US and Canada.12
A European registry (REDUCER-I, NCT02710435) is currently recruiting patients to a long-term study of the Neovasc Reducer™ system in patients with refractory angina pectoris. This registry encompasses 40 international centres and almost 100 of the planned 400 participants have been recruited to date. Preliminary data suggest improvement in CCS class and 6-minute walk test.12 Interestingly, 15–20 % of patients show no improvement with CSR (non-responders) and work is underway to identify potential mechanisms for this, with a recent study suggesting that non-responders have better-developed alternative venous drainage systems and achieve lower pressure rises in the coronary sinus during occlusion.19
While the CSR procedure is still in its relative infancy, its adoption into routine clinical practice is likely due to further positive clinical evidence and dissemination of specialist interventional expertise. The frequency of implantation currently varies between centres: an Italian centre reported five cases over 9 months, whereas a single-centre retrospective Dutch registry found 23 patients implanted with the CSR in a single year (2014).20,21 Furthermore, it has been suggested that the CSR may be used for a range of indications beyond RA. These include conditions with chronic chest pain, such as syndrome X and hypertrophic cardiomyopathy, as well as microvascular dysfunction. Studies to investigate this device in these patients are needed. A recent series of eight patients with microvascular dysfunction demonstrated significant improvements in CCS class (p=0.014), quality of life on SAQ (p=0.018) and 6-minute walk test (p=0.018) following CSR, with a subgroup (n=3) demonstrating improved myocardial perfusion reserve index.22 However, this was a small open-label case series with no control group and was non-randomised in design.
In chronic angina, patients can be revascularised by intervention of chronic total occlusions (CTOs). This method of improving arterial blood flow is often-lengthy, technically challenging and requires skilled operators. It is not without complications, although these have significantly reduced over time to rates comparable to non-CTO percutaneous coronary intervention.23 Nevertheless, there is still debate regarding the clinical efficacy of CTO at improving patient-related outcomes such as angina frequency, physical limitation and quality of life, given the conflicting results of recent CTO studies.24,25 Furthermore, CTO interventions do not offer any prognostic benefit but improve symptoms, which the CSR is also able to achieve. Consequently, an alternative for patients with occluded left coronary arteries – or those who have failed CTO procedures – may be the CSR, which is implanted in a shorter (~45-minute), easier procedure. Further studies to compare CSR against CTO are warranted.
External Enhanced Counterpulsation
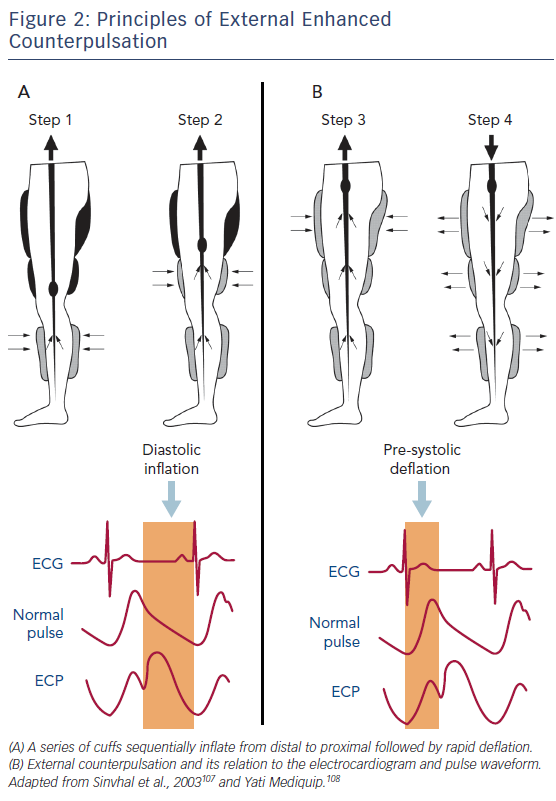
This non-invasive therapy involves placing external compressive cuffs on the calves, lower and upper thighs and then sequentially inflating them from distal to proximal in time with the cardiac cycle. Producing an effect similar to that of an intra-aortic balloon pump, the cuffs are inflated in early diastole to improve coronary perfusion and venous return, and deflated in systole to reduce systemic vascular resistance, improving cardiac workload and systemic perfusion (Figure 2). Treatment is performed in 1–2 hour sessions over a number of weeks, totalling approximately 35 hours in total. The mechanism of action through which counterpulsation improves coronary perfusion has been examined in a number of small studies that have also reported improved clinical outcomes.26 EECP has been shown to improve endothelial function,27 collateral flow and fractional flow reserve,28,29 to reduce arterial stiffness30,31 and to promote peripheral flow-mediated dilatation, as well as affecting endothelial-derived vasoactive agents by increasing nitric oxide turnover and reducing pro-inflammatory cytokines.31–33 It has also been shown that EECP up-regulates circulating CD34+ and CD133+ stem cell populations.34
The International EECP Patient Registry investigated patients with heart failure (ejection fraction of ≤35 %) with RA and found significant improvements in angina class (p<0.001), nitroglycerin use and quality of life after completion of treatment. These effects were maintained in a substantial proportion of patients at 3-year follow-up.35,36 However, the majority of studies of EECP have investigated its use in stable angina rather than exclusively in RA and, due to the nature of the intervention, blinding and control are difficult. Notably, the MUlticenter STudy of Enhanced External Counterpulsation (MUST-EECP), which included 139 participants, showed that time to ≥1 mm ST-segment depression improved significantly (~15 %, p=0.01) and angina frequency was reduced by 25 % compared to control (p<0.05).37 While meta-analyses have been encouraging, suggesting that about 85 % of patients with either refractory or chronic stable angina improve by ≥1 CCS class after EECP,38,39 a 2009 health technology assessment and 2010 Cochrane systematic review were unable to conclude clinical or cost-effectiveness, mainly due to poor trial design, lack of long-term follow-up, limited improvements in clinically-significant outcomes and an increased rate of adverse events.40,41 Consequently, the 2013 European Society of Cardiology guidelines for the management of chronic stable angina give EECP a Class IIa, Level of Evidence B recommendation for RA.42 The updated 2014 American Heart Association guideline for stable ischaemic heart disease made no change to the 2012 recommendation, which remains Class IIb, Level of Evidence B.43
EECP is limited by a number of contraindications, such as coagulopathy with an international normal ratio of >2.5, arrhythmias, severe peripheral arterial disease, venous disease and severe chronic obstructive pulmonary disease.26 In the UK, EECP is still in its infancy. There are currently only three active treatment centres and in 10 years fewer than 800 patients have been treated.44 However, EECP has been shown to reduce hospital costs.45 Growing interest, robust randomised clinical trials and evidence of cost-effectiveness will serve to encourage EECP adoption going forward.
Extracorporeal Shockwave Therapy
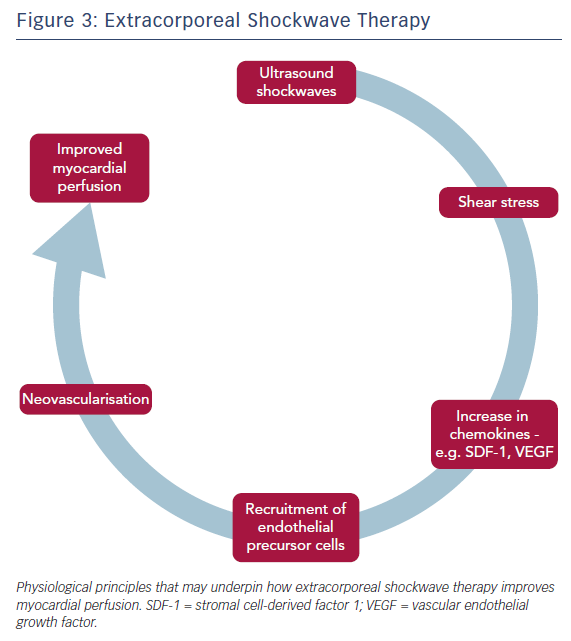
Another potential non-invasive therapy is ECSWT, which delivers low-energy shockwaves to the border zones of ischaemic myocardium and is guided by echocardiography (Figure 3). With the energy of such shockwaves typically being about 10 % of that delivered in urolithiasis, ECSWT is given in a series of sessions – typically over 4–9 weeks. It is a targetable treatment, unlike EECP, although both are able to treat right- and left-sided ischaemia. ECSWT is thought to induce neovascularisation. Animal studies have shown that ECSWT induces the development of collaterals and improves capillary density; recent data from clinical studies have reported improvements in myocardial perfusion.46–51 Pre-conditioning of chronic ischaemic tissue with ECSWT up-regulates chemoattractants such as stromal cell-derived factor 1 and vascular endothelial growth factor, improving the recruitment of endothelial precursor cells. In a clinical study, together with bone marrow-derived mononuclear cells (BMC), this recruitment results in modest but significant improvements in left ventricular ejection fraction (ECSWT + BMC: 3.2 %, 95 % CI [2.0–4.4 %] versus ECSWT + placebo infusion: 1.0 %; 95 % CI [−0.3–2.2 %]; p=0.02) and regional wall thickening (ECSWT + BMC: 3.6 %, 95 % CI [2.0–5.2 %] versus ECSWT + placebo infusion: 0.5 %, 95 % CI [−1.2–2.1 %]; p=0.01).50,52,53
To date, several randomised controlled studies in stable angina have demonstrated promising clinical outcomes without serious adverse effects.54 The largest study (n=45) to date showed that CCS class, NYHA class, myocardial perfusion, nitroglycerin usage, SAQ score, 6-minute walk test and left ventricular ejection fraction were all significantly improved at 3 months compared to controls as well as baseline (0 months). In the control group, none the above parameters had changed significantly from baseline at 3 months.55 A recent meta-analysis of ECSWT for angina was able to demonstrate significant improvements in angina class, SAQ score, nitrate consumption and exercise capacity. However, it should be noted that this study included single-arm, non-randomised and randomised trials and considered studies of chronic stable angina (the majority) as well as RA. Most trials were also small – the largest randomised, placebo-controlled study had a treatment arm consisting of 45 individuals – and they had varying durations of follow-up (range 1–72 months).54
ECSWT has been investigated specifically for RA in several studies, all of which suggest its efficacy and safety. A single-arm multicentre prospective study (n=111) of patients with RA undergoing ECSWT demonstrated improved summed difference score on stress SPECT (baseline 9.53±17.87; follow-up 7.77±11.83; p=0.0086), significantly improved SAQ score and nitroglycerin use.47 Recent data from the longest published follow-up (2.88±1.65 years) showed sustained reductions in CCS class (from 2.78±0.67 to 1.44±0.6; p=0.0002), nitroglycerin consumption (67 % versus 21. %; p<0.001) and also – importantly – reduced hospitalisation rate (40 % versus 18 %; p<0.03).56 Although progress has been made, ECSWT remains an experimental treatment. Its mechanism of action is uncertain and randomised controlled studies specific to RA are required. Indeed, the 2013 ESC guidelines on stable angina make little note of ECSWT, claiming that “more data are needed before establishing a potential recommendation”.57
Cell Therapy
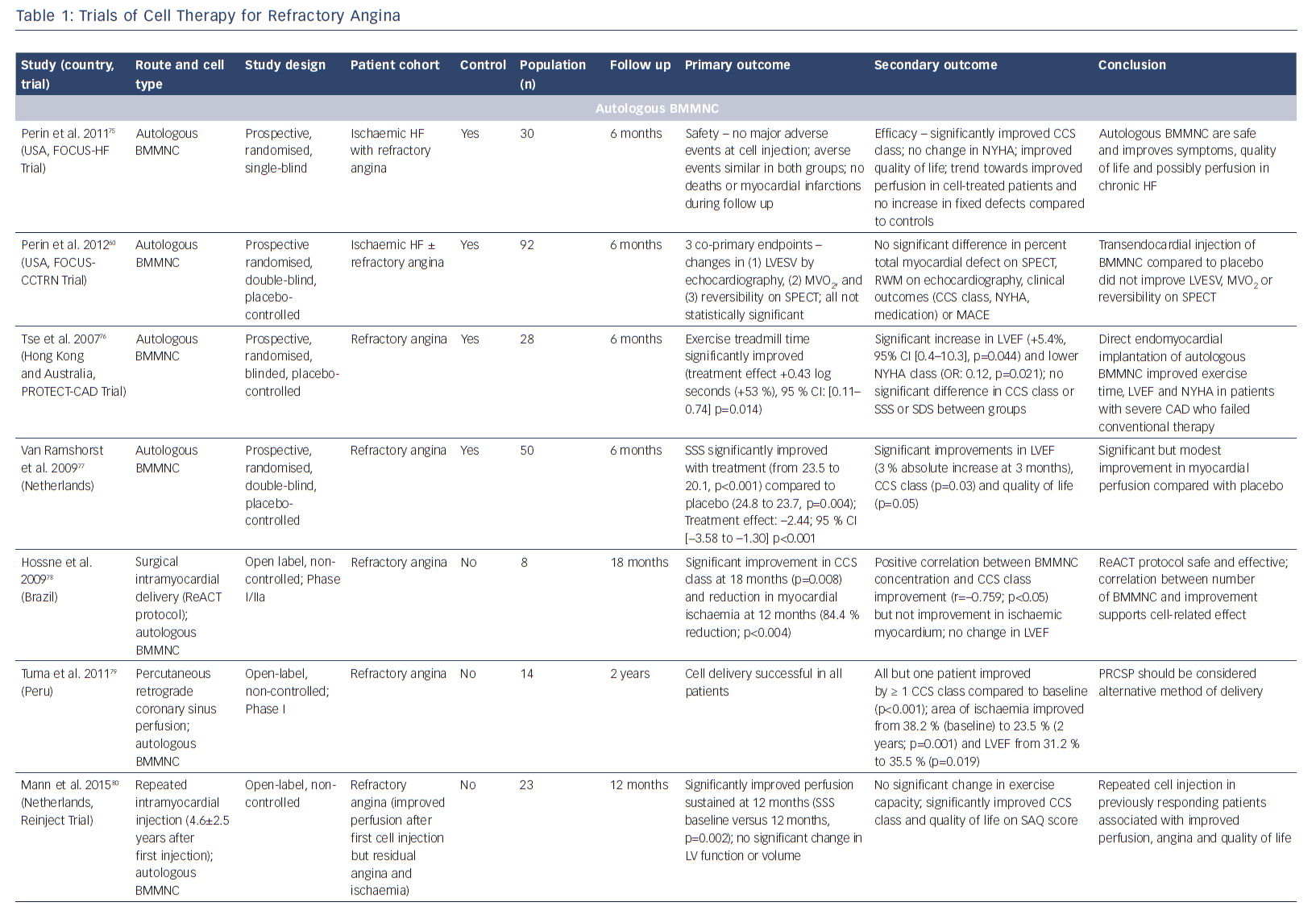
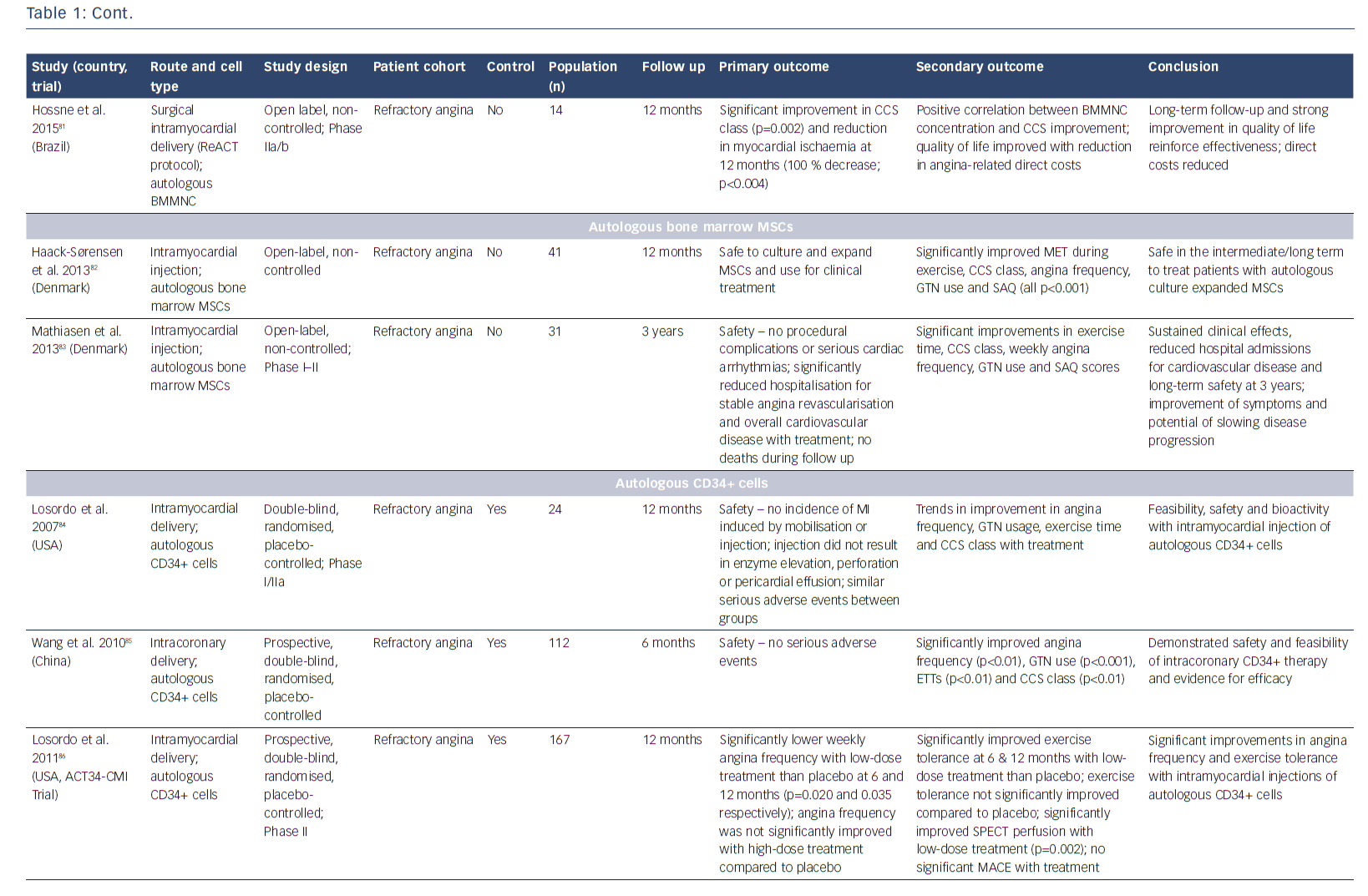
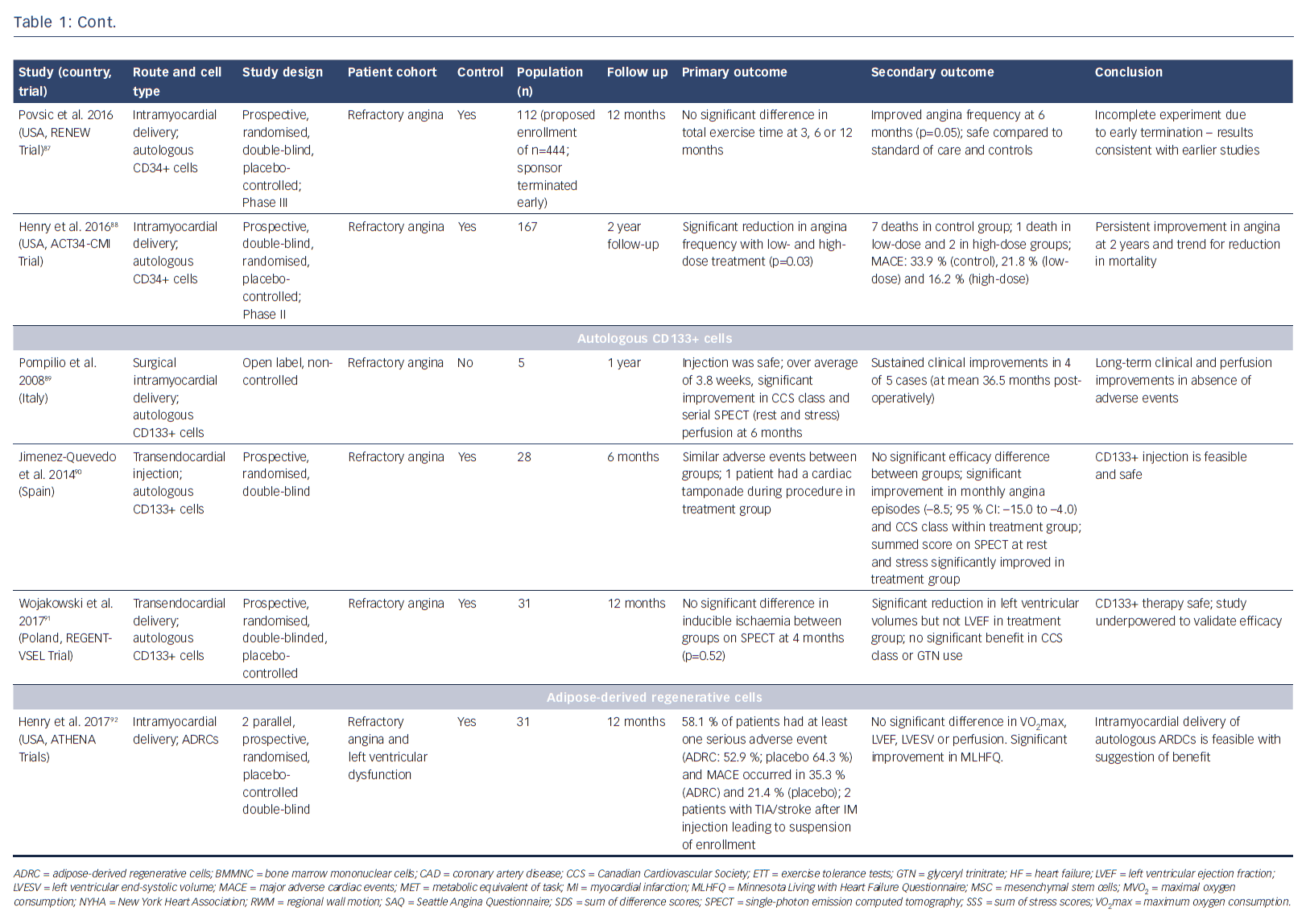
The potential of stem cells to protect, repair and regenerate the heart has been the subject of immense research over the past few decades. Researchers have investigated numerous cell populations, including unselected/selected bone marrow mononuclear cells,58–61 mesenchymal stromal cells,62 embryonic stem cells,63,64 induced pluripotent stem cells65 (pre-clinical studies only) and different populations of resident cardiac progenitor cells66-72 (see Table 2 in Madonna et al.73). It is increasingly recognised that these cell populations act through paracrine mechanisms to promote cardiac protection, repair and neovascularisation. While most reports have studied patients with acute myocardial infarction, as well as ischaemic and non-ischaemic heart failure, several studies have investigated the effects of cellular therapies for the treatment of patients with RA (Table 1).74–92
Bone marrow-derived CD34+ cells, which are considered to have haematopoietic and endothelial potential, have been shown to promote neovascularisation in ischaemic myocardium.93,94 Initial studies by Losordo et al. in a Phase I/IIa double-blind, randomised controlled trial demonstrated that intramyocardial injection of autologous CD34+ stem cells in patients with RA was feasible, safe and showed potential bioactivity with improvement in CCS class.84 The larger Phase II Adult Autologous CD34+ Stem Cells (ACT34-CMI) trial showed that intramyocardial injection of CD34+ cells into ischaemic but viable myocardium significantly lowered weekly angina frequency and improved exercise tolerance.86 Interestingly, the administration of high-dose CD34+ cells did not achieve a significantly greater therapeutic effect. At 2-year follow-up, both low- and high-dose groups had a significant reduction in angina frequency (p=0.03) and there was a trend towards improved mortality and major adverse cardiac events (MACE) (p=0.08 for both).88 On the basis of these promising results, the Efficacy and Safety of Targeted Intramyocardial Delivery of Auto CD34+ Stem Cells for Improving Exercise Capacity in Subjects with Refractory Angina (RENEW) trial was undertaken. This Phase III randomised, double-blinded, active-controlled standard of care trial included patients with RA, with CCS III or IV angina and ischaemia on stress testing. Randomisation was to three arms: cell therapy (granulocyte colony stimulating factor-mediated stem cell mobilisation, apheresis and intramyocardial injection of CD34+ cells); active control (granulocyte colony stimulating factor-mediated mobilisation, apheresis and intramyocardial placebo injection); or open-label standard of care.95 Primary efficacy was a change in exercise treadmill time. After recruitment of 112 out of the 444 planned patients, the study was terminated early due to strategic considerations. Although incomplete, the authors state that the results were consistent with earlier phase studies, demonstrating trends towards improved exercise time (improvement of 61 seconds with cell therapy at 3 months (p=0.06); 46.2 seconds at 6 months (p=0.22); and 36.6 seconds at 12 months (p=0.43)) and angina frequency at 6 months (p=0.05).95
Favourable outcomes have been demonstrated in studies with unselected and CD133+ bone marrow-derived stem cells.78,81,82 A long-term safety and efficacy study has demonstrated significant improvements in exercise time, CCS class, nitroglycerin use and SAQ score. Treatment also resulted in reduced hospital admissions and was demonstrated to have a good safety profile over a 3-year period.76 Small studies have investigated bone marrow-derived CD133+ cells. The recently-reported Intracardiac CD133+ Cells in Patients with No-option Resistant Angina (RegentVsel) trial (n=31) did not meet its primary endpoint of absolute change in myocardial ischaemia by SPECT compared to placebo; however, A Trial with Dronedarone to Prevent Hospitalization or Death in Patients with Atrial Fibrillation (ATHENA) recently suggested that the use of adipose-derived regenerative cells via intramyocardial delivery was feasible and should be investigated further for the treatment of RA (Table 1).91,92,96,97
Several meta-analyses have been performed on cell therapy to date.98-100 Collating data from five randomised controlled trials (n=381), Li et al. found that, compared to controls, cell therapy improved exercise tolerance by 61.3 seconds (p=0.005) reduced angina frequency by 7.3 episodes per week (p=0.02) and lowered the risk of myocardial infarction (OR 0.37, 95 % CI [0.14–0.95], p=0.04) with no difference in risk of death (OR 0.33, 95 % CI [0.08–1.39], p=0.13).98 The largest meta-analysis to date identified six randomised controlled trials (n=353) and concluded that cell therapies were safe but that they also improved angina frequency, use of anti-anginal medications, CCS class, exercise tolerance and myocardial perfusion and reduced MACE and arrhythmias compared to patients on maximal medical therapy.100 However, such publication-based meta-analyses should be interpreted with caution. Meta-analysis should ideally be performed using individual patient data, which although more arduous will reduce bias, e.g. from data analysis and reporting.101,102 By performing a meta-analysis on individual patient data from 1,275 people, Gyöngyösi et al. showed that effect sizes were much smaller than previously reported publication-based meta-analyses.103,104 We may now be seeing a move towards patient-level meta-analyses. A recent study by Henry et al. that pooled several studies of autologous CD34+ cells was encouraging, demonstrating meaningful improvements in total exercise time at 3, 6 and 12 months, and significantly reduced mortality at 24 months (12.1 % versus 2.5 %; p=0.0025).105
Limitations still exist and cell therapy remains an experimental treatment. Randomised controlled trials have been small and varied in design, with short follow-up periods. Questions remain, including optimal cell type, dosage, isolation and delivery methods. The duration of efficacy has also been questioned and researchers have suggested that repeated administration may be needed.80,106 The mechanism by which cell therapy is thought to improve clinical outcomes is also unclear. Suggestions that it promotes neovascularisation and improves microvascular and collateral perfusion are supported by an increase in myocardial perfusion on SPECT, although it must be noted that its reliability is limited in this population due to the common presence of multivessel disease. Consequently, larger Phase III studies are needed and should build on lessons learnt from previous studies. Clinically-relevant outcomes should be studied including quality of life, cost-effectiveness and MACE, and quantitative assessment of myocardial perfusion should consider the use of PET and cardiovascular magnetic resonance to overcome the limitations of SPECT, such as poor spatial resolution, long acquisition times and balanced flow reduction seen in multivessel disease.108
Conclusion
Significant progress has been made in developing novel treatments for patients with RA. Given the growing clinical burden that this condition represents, the need for such interventions is greater than ever. This paper discusses four major advances and considers the evidence supporting their use. While some remain experimental, the CSR and EECP are currently being used in clinical practice. Further robust clinical data and assessment of cost-effectiveness must be sought to aid their incorporation into guidelines. Nevertheless, with growing experience of their use and evidence from larger randomised controlled studies, these therapies hold much promise in treating patients with RA.