Cardio-oncology is an emerging field of cardiology that focuses on cardiovascular diseases in patients with cancer. The classic cardio-oncology paradigm is the prevention, diagnosis and treatment of cardiotoxicity resulting from chemotherapy and/or radiotherapy. Diagnosis and treatment of primary and metastatic cardiac tumours as well as cardiac amyloidosis can be considered ‘less classical’ cardio-oncology objectives.
Anthracycline-induced cardiotoxicity was first reported in early 1970s.1 Since then, there has been increasing recognition of its association with poor prognosis and survival.2 More recently, while new targeted and more effective molecules have been introduced in clinical oncology, cardiotoxic effects – which are not uncommon – may potentially outweigh theoretical clinical benefit.
The incidence of cardiotoxicity varies with the type of the treatment. Doxorubicin is associated with cardiotoxicity in 3–26 % of treated patients, trastuzumab in 2–28 % and sunitinib in 2.7–11 %.3 In a recent retrospective study, 6.6 % of patients with breast or haematological cancer who received chemotherapy went on to develop heart failure.4 Furthermore, patients with cancer are also at a higher risk for coronary artery disease, arrhythmias and thromboembolism.5 Interestingly, cancer and coronary artery disease share common cellular and genetic pathways as well as risk factor profiles.6
Overall, the management of patients with cancer is complex and requires a multidisciplinary team approach involving oncologists, surgeons, radiologists and clinical cardiologists. The aim of this review is to provide an overview of chemotherapy and radiotherapy-related cardiotoxicity, with a special focus on diagnosis, prevention and management.
Cardiovascular Complications
Anti-cancer treatment is associated with serious cardiovascular adverse events, including arterial and pulmonary hypertension, supraventricular and ventricular arrhythmias, systolic and diastolic cardiac dysfunction and coronary artery disease. It has been postulated that chemotherapy- and radiotherapy-related endothelial dysfunction, thrombogenesis and myocardial injury may partly explain these cardiovascular complications.
Myocardial Dysfunction
According to recent cardio-oncology expert consensus, significant cardiotoxicity after chemotherapy is considered when the following heart echocardiographic criteria are fulfilled: (i) an absolute decrease of ≥10 % in left ventricular ejection fraction (EF), and (ii) an EF of <50 %. Additionally, left ventricular global longitudinal strain (GLS) is proposed as an early marker of imminent cardiotoxicity because a reduction in GLS of >15 % during chemotherapy is associated with a higher probability of significant left ventricular systolic dysfunction in the near future.7
Two pathophysiological mechanisms have been described for chemotherapy-induced cardiotoxicity. First is direct toxicity and destruction of myocardial cells, which results in permanent and possibly irreversible myocardial dysfunction (type I cardiotoxicity). The second is inhibition of the physiological function of myocardial cells, which results in ‘stunned’ myocardium and significant but eventually reversible myocardial dysfunction (type II cardiotoxicity).8 These two mechanisms frequently overlap. The classic example of type I cardiotoxicity is anthracycline cardiotoxicity, which is usually dose dependent. Trastuzumab cardiotoxicity is an example of type II cardiotoxicity but it is not dose-dependent.9
Myocardial Ischaemia
Some chemotherapeutic agents (i.e. 5-fluorouracil and gemcitabine) increase the risk of coronary atherosclerosis and acute coronary syndromes. Continuous intravenous infusion of 5-fluorouracil can induce myocardial ischaemia that manifests as chest pain and ischaemic ECG changes, usually between the second and fifth day of treatment. This effect is not dose dependent.10 The pathophysiological mechanisms implicated are vasculitis, spasm and thrombosis. VEGF inhibitors such as bevacizumab and cisplatin have also been associated with myocardial ischaemia through endothelial dysfunction, hypercoagulability and thrombosis.28 The incidence of cisplatin-associated acute coronary syndrome is approximately 2 %.7
Radiotherapy is associated with coronary atherosclerosis – especially in the coronary ostia – and a higher risk for acute coronary syndromes. Following radiotherapy for Hodgkin lymphoma, the cumulative incidence of coronary artery disease is high (approximately 20 %), even 40 after years.38 Therefore, long-term follow-up and close monitoring for several years after radiotherapy is reasonable.
Interestingly, coronary artery disease and cancer share similar risk factors and pathophysiological pathways (i.e. chronic inflammation). Modification of risk factors has been shown to prevent the development of both coronary artery disease and cancer but little is known about its effect on chemotherapy and/or radiotherapy-induced coronary cardiotoxicity.6
Arterial Hypertension
Arterial hypertension is frequently reported in patients receiving VEGF inhibitors (11–45 %). Bevacizumab and sunitinib increase the risk of arterial hypertension or aggravation of pre-existing hypertension – possibly via inhibition of angiogenesis, reduction in nitric oxide and increase in endothelin-1 levels along with glomerular injury and renal microangiopathy.11 Furthermore, the inhibition of beta-type platelet-derived growth factor receptor by sunitinib has been associated with microcirculatory dysfunction.
Consequently an acute rise in arterial blood pressure – even in normotensive patients – can be expected following the introduction of VEGF inhibitors, and regular blood pressure recordings and blood pressure adjustment with antihypertensive medications are generally recommended. A significant increase in arterial blood pressure is normally observed in the first year after treatment. Angiotensin-converting-enzyme (ACE) inhibitors and calcium channel blockers are usually prescribed in these cases.12
Arrhythmias and Anticoagulation in Atrial Fibrillation/Flutter
Arrhythmias – either supraventricular or ventricular – can frequently occur during chemotherapy. It has been shown recently that a non-negligible number of patients with chronic lymphocytic leukaemia treated with ibrutinib developed AF (approximately 3 %).13 In contrast, thalidomide is associated with an increased risk of bradyarrhythmias and therefore beta-blockers and calcium channel blockers should be used with caution in these cases. Arsenic trioxide, a very effective drug for relapsing acute promyelocytic leukaemia, may prolong the QT interval and induce torsades de pointes.14 Thus, QT interval should be carefully monitored in patients receiving arsenic trioxide before every new cycle of therapy. Less frequently, tyrosine kinase inhibitors, proteasome inhibitors and histone deacetylase inhibitors may prolong QT.14
Anticoagulation in patients with cancer and AF can be difficult to manage. Cancer is often associated with a higher risk for thrombosis, but at the same time cancer therapies may predispose to a higher risk for bleeding. The scores commonly used to evaluate embolic (CHADS-VASC) and bleeding risk (HAS-BLED) in patients with AF are possibly not applicable in patients with coexistent AF and malignancy.7 Furthermore, there are no robust data on the safety and efficacy of both vitamin K antagonists and the new oral anticoagulants during or after chemotherapy – especially in patients with imminent thrombocytopaenia. For this reason, decision making for anticoagulation in patients with malignancy and AF should be individualised. Low molecular weight or classical heparin might be an alternative short-term anticoagulation option.15
Pulmonary Hypertension
Dyspnoea secondary to pulmonary hypertension is a relatively frequent adverse effect of dasatinib, a chimeric oncogene BCR-ABL tyrosine kinase inhibitor used for the treatment of chronic myeloid leukaemia. The underlying pathophysiological mechanism is not clear, although toxicity is usually reversible after dasatinib discontinuation.16 Pulmonary arterial hypertension has also been sporadically reported with thalidomide and carfilzomib.17,18
Thromboembolic Disease
Malignancy is known to be associated with a prothrombotic milieu, which may be exacerbated by chemotherapy. Immunomodulatory imide drugs such as thalidomide, lenalidomide and pomalidomide commonly used in the treatment of multiple myeloma are associated with a risk for thromboembolism, ranging from 10 to 40 %. Both patient- and drug-related factors have been implicated in this variability.19 The prophylactic use of aspirin for low-risk patients and anticoagulation with either low molecular weight heparin or warfarin for high-risk patients is generally recommended.15,20 Cisplatin, erlotinib and bevacizumab have also been reported to increase the risk for thrombotic events but there are no special recommendations for thrombosis prophylaxis.15
Valvular Heart Disease
Autopsy studies have suggested that mediastinal irradiation for a wide range of cancers may potentially adversely affect the heart valves.21 Diffuse or focal fibrosis, thickening and calcification of the valves as a result of upregulation of fibrogenic growth factors, and increased formation of osteogenic factors have been described. Interestingly, in contrast to rheumatic valve disease, radiation does not usually affect the tips of the valves. The prevalence of radiation-induced valve disease ranges from 2 to 37 % for Hodgkin lymphoma and from 0.5 to 4.2 % for breast cancer.21 A long latent interval between radiation exposure and valve disease (usually >10 years) has been reported.21 It is important to note that in immunocompromised patients a high level of awareness of infective endocarditis is particularly warranted, especially when valve regurgitation is discovered during or after chemotherapy. Finally, chemotherapy-induced cardiotoxicity may associate with severe functional mitral valve regurgitation, which should be promptly diagnosed and treated.
Pericarditis and Pericardial Effusion
Both radiotherapy and chemotherapy (i.e. anthracyclines, bleomycin, cyclophosphamide) can be associated with a chronic inflammatory process of the pericardium. Radiation-induced pericardial effusion has been reported as late as 15 years following radiotherapy.22 Another manifestation of pericardial disease is constrictive pericarditis, which might develop after exposure to high radiation doses.23
Peripheral Artery Disease
Tyrosine kinase inhibitors (nilotinib and ponatinib) may adversely affect the peripheral arterial circulation and increase the risk of peripheral artery disease even in the absence of traditional cardiovascular risk factors.12 Previous neck irradiation increases the chance of acceleration of carotid artery atherosclerosis and ischaemic stroke.21
Cancer Treatments Associated with Cardiotoxicity
Anthracyclines
Anthracycline-associated cardiotoxicity is traditionally divided into early-onset acute toxicity and late-onset chronic evolving toxicity. Early-onset cardiotoxicity usually occurs within hours to weeks but definitely during the first year after anthracycline administration, and can be reversible with early detection and treatment.24 Late-onset cardiotoxicity can present in a period of 10–20 years after treatment. The clinical presentation of acute toxicity is variable, ranging from arrhythmias and myocarditis to acute coronary syndromes and acute heart failure.25 The molecular mechanism of anthracycline-induced cardiotoxicity involves the inhibition of topoisomerase IIb in myocardial cells, which induces DNA double-strand breaks and activation of the apoptotic programme of the heart via mitochondriopathy and increase in radical oxygen species.26 The most commonly used anthracyclines are doxorubicin, epirubicin and idarubicin. The highest doxorubicin cumulative dose recommended is 400–550 mg/m2.27 Higher doses significantly increase the risk of cardiotoxicity, which can range from 18 to 48 % for a cumulative dose of 700 mg/m2.7 However, even low doses (<300 mg/m2) are associated with a non-negligible risk for cardiotoxicity (1.6 %). Liposomal anthracyclines such as pegylated liposomal doxorubicin are significantly less cardiotoxic and have comparable effectiveness, and can be an alternative choice for patients at high risk for cardiotoxicity.28
HER2 Inhibitors
The human epidermal growth factor receptor (HER) 2 inhibitor trastuzumab has proven to be very effective in metastatic HER2-positive breast cancer. However, HER2 is also expressed on cardiomyocytes and its inhibition by trastuzumab can possibly lead to the development of cardiotoxicity.29 Trastuzumab cardiotoxicity is often reversible with cessation of therapy and initiation of heart failure medications. However, some degree of persistent myocardial dysfunction is documented in nearly one third of these patients.30 Re-introduction of the medication should be always balanced against cardiotoxicity risk.30 Concomitant use of trastuzumab and anthracyclines is associated with a higher risk of cardiotoxicity compared with anthracycline monotherapy.31 Other approved anti-HER2 agents, such as pertuzumab and trastuzumab emtansine, are also considered cardiotoxic.31
VEGF Inhibitors
It is difficult to make an accurate estimate of vascular endothelial growth factor (VEGF) inhibitor-related cardiotoxicity as large clinical trials are lacking. In general, sunitinib has been found to cause systolic dysfunction in 3–15 % of patients and bevacizumab-associated heart failure is observed in 2 % of patients.7,32 A recent meta-analysis showed a higher risk for congestive heart failure with anti-VEGF molecules, although severe heart failure was rare.33 In murine models, one possible mechanism explaining VEGF inhibitor-related cardiotoxicity is via myocardial capillary rarefaction, which induces hypoxia and activation of hypoxia-related genes and apoptosis.20
Proteasome Inhibitors
Proteasome inhibitors inhibit different catalytic sites of proteasome and through caspase 3/7 signalling result in apoptosis and left ventricular systolic dysfunction.35 Bortezomib is the first proteasome inhibitor clinically tested in haematological malignancies, and acts via reversible inhibition the 26S site of proteasome. It has been estimated that it might double the risk for all-grade cardiotoxicity in multiple myeloma patients.35 Carfilzomib is a novel proteasome inhibitor approved by the Food and Drug Administration for multiple myeloma resistant to bortezomib. It irreversibly inhibits the beta 5 subunit of the 20S proteasome complex and is considered more cardiotoxic than bortezomib. Carfilzomib has also been associated with a higher incidence of cardiac arrhythmias. Ixazomib is the first oral proteasome inhibitor that reversibly inhibits the beta 5 subunit of the 20S proteasome complex, and only sporadic cases of cardiotoxicity have been reported.35
Other Commonly used and Potentially Cardiotoxic Treatments
Cyclophosphamide- and ifosfamide-induced cardiotoxicity is dose dependent and usually presents few days after treatment. High doses (>140 mg/kg) are considered very cardiotoxic. Although platinum-based therapies have been associated with decompensated heart failure, pre-existing myocardial dysfunction and volume overload during platinum administration are probably responsible for heart failure decompensation rather than platinum per se.36 New immunotherapy agents such as ipilimumab (an anti-cytotoxic T-lymphocyte-associated protein 4 monoclonal antibody) and nivolumab (an anti-programmed death 1 monoclonal antibody) are usually co-administered for the treatment of melanoma and have been estimated to induce resistant myocarditis in approximately 1 % of patients.37 Interestingly, several chemotherapeutic agents have been linked with left ventricular diastolic dysfunction, with a benign prognosis in the majority of cases.
Radiotherapy
The relative risk for the development of heart failure in the context of radiotherapy is difficult to estimate with accuracy because the co-administration of cardiotoxic chemotherapy is a confounding factor. However, it should be emphasised that patients with breast cancer treated with both chemotherapy and radiotherapy are at increased risk for developing cardiotoxicity compared with chemotherapy alone. There is often a long delay between exposure to radiation and clinically apparent heart dysfunction. The development of significant fibrosis post radiotherapy that can potentially affect all cardiac structures (myocardium, pericardium, coronary arteries or heart valves) has been described widely in the literature.38 Of note, radiotherapy-induced coronary or valvular heart disease may also progress after years to congestive heart failure.39,40
Diagnosis and Treatment
Initial Evaluation
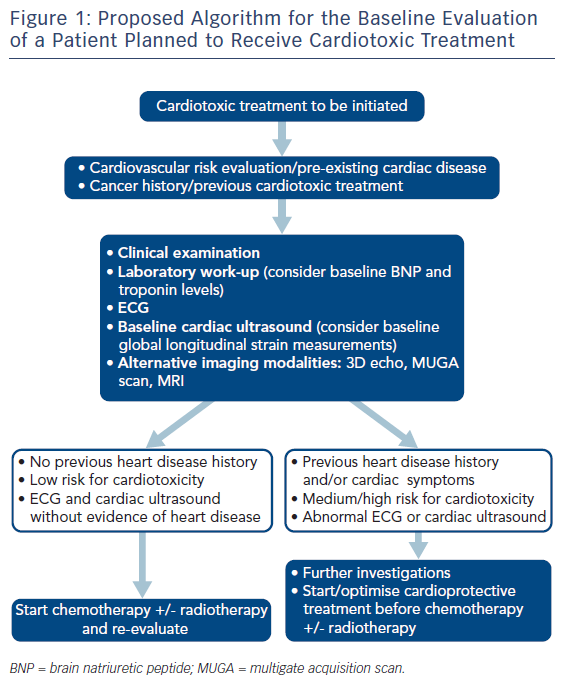
A baseline work-up including medical history, clinical examination, laboratory tests, an electrocardiogram and an echocardiogram with speckle tracking should precede a potentially cardiotoxic chemotherapy (Figure 1).6,41 Predictive models can be used to assess the risk of cardiotoxicity on the basis of the baseline patient characteristics. Factors that may predispose to cardiac adverse events include a previous diagnosis of structural heart disease, the presence of traditional cardiovascular risk factors, previous or future exposure to cardiotoxic therapy and older age (Table 3).
Other baseline tests, such as 24–hour ECG and 24-hour blood pressure monitoring, functional ischaemia testing, magnetic resonance imaging and right heart catheterisation, can also be considered for diagnosis and/or risk stratification. Cardiac biomarkers such as high sensitivity troponin I, brain natriuretic peptide (BNP) and NT-proBNP may be helpful in identifying patients at risk for cardiotoxicity. However, limited data exist on the timing for these tests and their clinical impact on further patient management.42
Imaging and Monitoring for Systolic Dysfunction
At present, the most commonly used parameter for the evaluation of the systolic function is the EF. However, intra-observer and inter-observer variability for 2D EF measurements using the Simpson’s biplane method is not negligible. In addition, small but significant EF changes (≤10 %) might be missed by 2D but usually not by 3D echo.43 Obstacles such as poor image quality resulting from rapid heart rate, increased cardiac translocation, and respiratory interference might be overcome with intravenous contrast infusion.
Radionuclide ventriculography or multigate acquisition scan (MUGA) is another relatively accurate and alternative to heart echo imaging modality to detect even asymptomatic left ventricular dysfunction in patients receiving chemotherapy. MUGA has been previously used to evaluate left ventricular function after anthracycline administration, and many of the recommendations for the monitoring and management of anthracycline-induced cardiotoxicity are based on MUGA findings.44 Cardiac MRI is another imaging modality that can precisely identify early signs of cardiotoxicity (i.e. inflammation and oedema) and assess ventricular volumes and function. The extent of late gadolinium enhancement may identify patients with worse prognosis, whereas diffuse fibrosis in T1 mapping can predict late anthracycline cardiotoxicity.45 Furthermore, cardiac MRI is probably the best technique for the diagnosis of cardiac tumours and radiotherapy-related pericardial disease.
Follow-up and Treatment of Myocardial Dysfunction
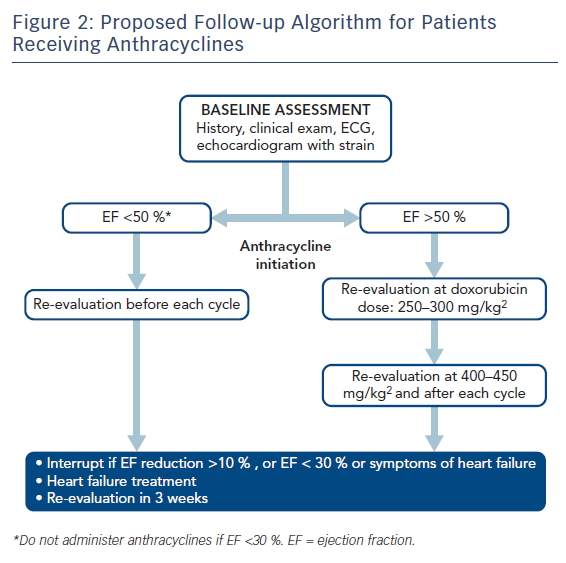
For patients receiving anthracyclines and/or trastuzumab after baseline clinical evaluation and heart echo, routine clinical follow-ups and heart echos are recommended every 3 months for the first year of therapy.41 For anthracyclines in particular, a repeat echo is also advisable at a cumulative dose of 240 mg/m2 or even earlier if clinical symptoms and/or an increase in cardiac enzymes is observed (Figure 2). For higher anthracycline doses, routine echocardiograms are recommended before each anthracycline cycle. For less cardiotoxic chemotherapy, follow-up assessments should be individualised.
Troponin levels at the beginning of the treatment and after each cycle might be helpful in the early identification of patients at high risk for cardiotoxicity.46 Any troponin increase should be discussed in a multidisciplinary team meeting with oncologists and cardiologists to set the onward treatment plan for the patient. In patients with breast cancer, troponin and GLS measurements are the best predictors of cardiotoxicity with a specificity of 93 % and a negative predictive value of 91 %.47
Long-term clinical follow-up is mandatory for anthracyclines. Routine heart echos are recommended at 6, 24 and 36 months after the last anthracycline cycle. In particular, for patients who received anthracyclines before adolescence (<15 years old) or those exposed to high doses (>240 mg/m2), follow-up monitoring should extend to 4 and probably 10 years after therapy.13 Anthracyclines are contraindicated in patients with baseline severe systolic dysfunction (EF <30 %). A strong indication for temporary cessation of anthracyclines is an absolute EF decline of >10 %.43
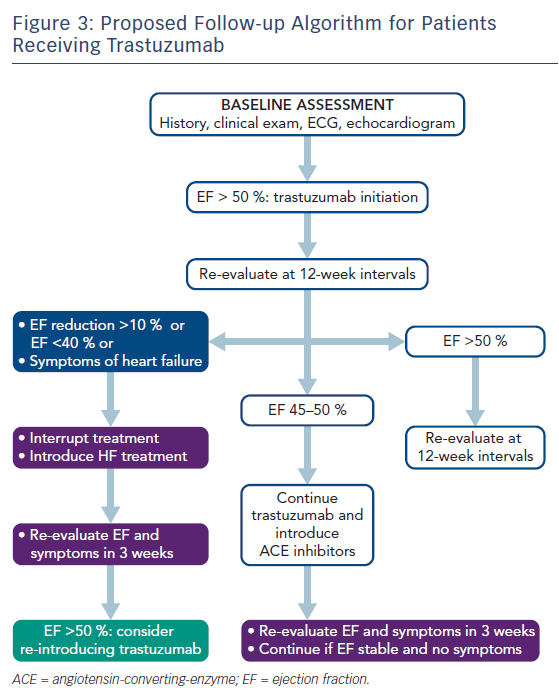
In patients receiving trastuzumab, the detection of a mildly decreased EF (45–50 %) after therapy advocates both the introduction of heart failure treatment and re-assessment after 3 weeks without modification of trastuzumab dose (Figure 3). However, trastuzumab should be discontinued when EF drops below 45%, or to 45–50 % with an absolute decrease of at least ≥10 % compared with baseline.48
In patients receiving VEGF inhibitors, the optimal follow-up strategy remains unclear. However, it is reasonable to schedule a routine clinical examination and heart echo after 2–4 weeks of treatment, especially in patients with high risk criteria for developing cardiotoxicity.7
The prophylactic prescription of beta-blockers and ACE inhibitors before chemotherapy is generally reserved for high-risk patients (i.e. previous administration of anthracyclines/trastuzumab, baseline EF <50 %) although data are still controversial.46 In contrast, the prophylactic use of dexrazoxane is strongly supported for the prevention of cardiotoxicity in breast cancer patients treated with high doses of doxorubicin (>300 mg/m2).27
Treating Myocardial Ischaemia in Patients with Cancer
The management of acute coronary syndromes in patients with cancer is challenging. A recent study from the Mayo Clinic has shown that 10 % of patients presenting with ST-elevation MI (STEMI) are patients with cancer. Interestingly, primary percutaneous coronary intervention results in similar survival benefit in those patients compared with the general population with STEMI.49 The administration and duration of dual antiplatelet therapy must be individualised after balancing ischaemic and bleeding risk. The type of cancer and its prognosis and management should also be taken into account before dual antiplatelet therapy is given. In general, the duration of dual antiplatelet therapy should be the shortest time possible. In patients with severe thrombocytopaenia (platelet count <50.000/μL) the bleeding risk increases exponentially. According to current guidelines, dual antiplatelet therapy can be considered with caution when platelet count is higher than 30.000/μL, whereas aspirin as monotherapy is recommended when platelet count is between 10.000 and 30.000/μL.50 Radial access is associated with less bleeding and better clinical outcomes in patients with acute coronary syndromes. Extrapolating these findings in patients with cancer, a radial approach must be the preferred route for these patients too, because their bleeding risk is much higher than in the general population.
Prevention and Follow-up
Oncology patients at high risk for cardiotoxicity should be referred and assessed by cardiologists before chemotherapy. Less cardiotoxic chemotherapeutic agents and anti-remodelling drugs such as a beta-blockers, ACE inhibitors or angiotensin receptor blockers before chemotherapy may theoretically prevent cardiotoxicity in high risk patients. Of the beta-blockers, both nebivolol and carvedilol have been studied for the prevention and treatment of cardiotoxicity.46 However, there is equivocal evidence for the prophylactic use of some beta-blockers, ACE inhibitors and angiotensin receptor blockers in the recent literature. Pituskin et al. showed that perindopril and bisoprolol attenuated cardiotoxicity in patients treated with trastuzumab, but not left ventricular remodelling.51 In contrast, enalapril prevented cardiotoxicity in patients receiving anthracyclines as evidenced by the extent of myocardial injury measured with troponin.52 In the Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) trial, candesartan but not metoprolol prevented the development of systolic dysfunction in breast cancer patients receiving anthracyclines.53 Conversely, Boekhout et al. showed that candesartan was not superior to placebo in preventing cardiotoxicity in breast cancer patients treated with anthracyclines and trastuzumab.54
Data from retrospective studies suggest that statins may prevent the development of heart failure, but prospective data are warranted to confirm this.55 As already discussed, dexrazoxane may attenuate the cardiotoxic effect of anthracyclines, possibly via a significant reduction of superoxide radicals produced by anthracyclines. Interestingly, it has been suggested that the modification of traditional risk factors together with regular aerobic exercise may prevent cardiotoxicity.56
Conclusion
Cardio-oncology is increasingly becoming part of routine clinical practice. Alongside oncologists and cardiologists it requires the collaboration of several medical specialties, with this multidisciplinary approach contributing to improved outcomes in cancer patients in the contemporary era. The goal of cardio-oncology is to accomplish the prevention, early recognition and management of cardiotoxicity, but there are still a number of questions to be answered to improve patient prognosis and quality of life. On-going and future research is expected to elucidate the exact mechanisms involved in chemotherapy-induced cardiotoxicity and to accurately identify the genetic and molecular profiles underlying those mechanisms and making the heart more vulnerable to chemotherapy.