Professor Maseri was a pioneer who blazed a trail in the field of coronary vasomotion abnormalities from its dawning age and was one of the most prominent physician-scientists represented in the history of the field.1,2 More than 40 years ago, he demonstrated that myocardial ischaemia can be caused by coronary vasomotion abnormalities, such as coronary vasospasm and distal coronary constriction, even in the absence of fixed obstructive coronary artery disease.3–5 Moreover, he showed that coronary vasospasm can be relieved by treatment with calcium-channel blockers, a drug of choice for contemporary vasodilator therapy in patients with vasospastic angina.6 Revascularisation of the culprit lesion has long been a cornerstone of modern care for ischaemic heart disease because of easy visibility on coronary angiography and amenability to procedural interventions by means of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). However, approximately half of patients with signs and symptoms suggestive of myocardial ischaemia do not have obstructive, flow-limiting coronary artery stenosis on coronary angiography.7–16 The term ischaemia with non-obstructive coronary artery disease (INOCA) has been coined to define patients with this particular condition.17,18 The major mechanisms of myocardial ischaemia in patients with INOCA include coronary vasospasm and coronary microvascular dysfunction (CMD), the latter of which manifests itself as the structural and functional abnormalities of the coronary microvasculature, including coronary microvascular spasm.18,19 CMD is more prevalent than previously thought in many clinical settings, associated with worse clinical outcomes.12,13,20,22–33 A large-scale nationwide survey in the US evaluating a total of 12,062,081 coronary revascularisations revealed that risk-adjusted mortality did not decrease after PCI across all clinical indications.34 In line with these results, a questionable benefit of revascularisation by PCI or CABG versus optimal medical therapy in patients with stable coronary artery disease has been replicated by the findings of the two landmark clinical trials, the ORBITA trial and the ISCHEMIA trial.35,36 A recent post-hoc analysis of the ISCHEMIA trial showed that the prevalence of INOCA was at least 13% (476/3,612) in the participants with moderate or severe myocardial ischaemia, who were characterised by younger age, female preponderance, and lower atherosclerotic burden.37 Notably, the severity of ischaemia was not associated with that of non-obstructive coronary atherosclerosis.37 Although these trials did not primarily focus on coronary microvascular function, a fascinating speculation is that coronary vasomotion abnormalities, such as coronary vasospasm and CMD may contribute to myocardial ischaemia even after successful revascularisation, as predicted previously by Professor Maseri.38 This review will highlight the pioneering works of Professor Maseri and cutting-edge research on CMD from bench to bedside, with focus placed on the clinical implications of coronary microvascular spasm.
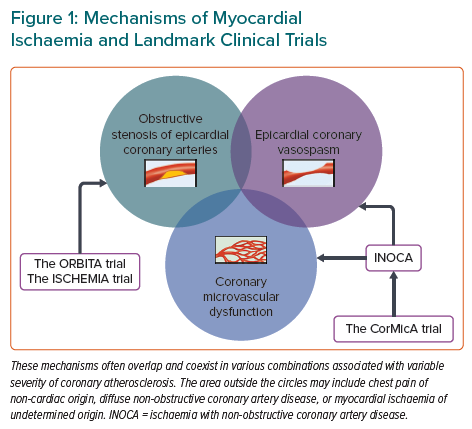
Mechanisms of Myocardial Ischaemia
The coronary circulation consists of epicardial conductive arteries (>500 μm in diameter), prearterioles (100–500 μm), arterioles (<100 μm), capillaries, and veins.39–41 Only epicardial coronary vessels are visible on coronary angiography, although most of the coronary resistance is determined by the coronary microcirculation. The mechanisms of myocardial ischaemia are three-fold: obstructive, flow-limiting stenosis of epicardial coronary arteries; epicardial coronary vasospasm; and CMD (Figure 1).19,42,43 The latter two mechanisms have been acknowledged as the major culprits of myocardial ischaemia in INOCA, although it took many years for the cardiovascular scientific community to accept the idea of vasospastic myocardial ischaemia in patients with variant angina.18,19,44–46 Armed with radioisotope techniques for measuring coronary blood flow, Professor Maseri proved that epicardial coronary vasospasm is a cause of spontaneous angina at rest in patients with variant angina.47–57 This proposal initially faced many criticisms because it contradicted the prevailing dogma that myocardial ischaemia was solely attributable to fixed obstructive coronary artery disease.46,58,59 Indeed, coronary vasospasm was once defined as “the resort of the diagnostically destitute” based on the pathological notion that atherosclerotic coronary arteries could not constrict.59 Nonetheless, an expanding body of evidence has demonstrated that coronary functional abnormalities manifested as coronary vasospasm and CMD play distinct roles in the pathophysiology of myocardial ischaemia across a wide spectrum of cardiovascular diseases.19,41,44,60–62 In the 2019 European Society of Cardiology Guidelines for the diagnosis and management of chronic coronary syndromes (CCS), patients with angina and suspected vasospastic or microvascular disease are classified as CCS V, which is one of the most frequently encountered clinical scenarios in patients with CCS.63
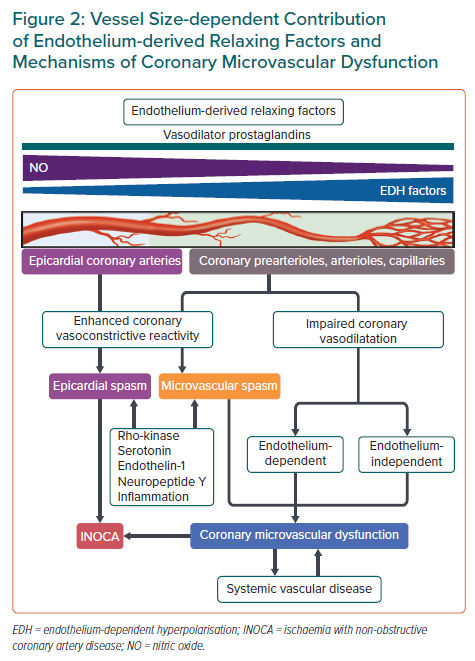
Enhanced coronary vasoconstrictive reactivity at epicardial and microvascular levels causes a transient decrease in coronary blood flow, resulting in supply ischaemia or primary angina (Figure 2).54,64 Coronary microvascular spasm is one of the mechanisms responsible for myocardial ischaemia in patients with microvascular angina (MVA).65 On the other hand, impaired endothelium-dependent and -independent coronary vasodilator capacities as well as elevated coronary microvascular resistance secondary to structural factors (e.g. luminal narrowing, vascular rarefaction, vascular remodelling and extramural compression) cause myocardial ischaemia when oxygen consumption is increased in the same way as obstructive coronary stenosis, leading to demand ischaemia or secondary angina (Figure 2).54,64 These underlying mechanisms often overlap and coexist in various combinations associated with variable degrees of coronary atherosclerosis, making the clinical picture of patients with INOCA highly heterogeneous (Figure 1).13,30,66,67 Accordingly, comprehensive assessment of coronary vasomotor reactivity by invasive functional coronary angiography or interventional diagnostic procedure using vasoactive agents (e.g. acetylcholine and adenosine) is recommended to identify the underlying mechanisms of myocardial ischaemia and to tailor the most appropriate treatment and management based on the endotype of INOCA.18,45 A good example of this tailored therapeutic strategy is provided by the CorMicA trial demonstrating that a stratified medical treatment driven by the results of coronary reactivity testing is beneficial in patients with INOCA.14 The provisional notion of Professor Maseri is certainly pertinent that a pathogenetic classification of angina may be helpful for the management of patients with the disease.54,64
Clinical Presentation of Coronary Microvascular Spasm
Coronary microvascular spasm may manifest symptomatically as MVA.65 Patients with this condition may present with typical angina, angina equivalents, such as dyspnoea and fatigue, and other atypical symptoms occurring not only at rest but also on exertion. In some cases, coronary microvascular spasm may be identified in asymptomatic subjects.65 Patients with coronary microvascular spasm are characterised by predominance of postmenopausal women, fewer coronary risk factors including smoking, and longer duration of angina.30,65,68–73 Although the prognosis of patients with MVA has been considered to be benign in most cases, the symptoms are often refractory to conventional treatment with calcium-channel blockers alone, leading to impaired quality of life.30,69,72–75 A large-scale (n=686), international, prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group recently showed that microvascular angina, in which coronary microvascular spasm was the most frequent aetiology (42%), is a considerable health problem regardless of sex or ethnicity and associated with major adverse cardiovascular events driven by hospitalisation due to unstable angina.76 The prevalence of coronary microvascular spasm ranges from approximately 20–50% in patients with INOCA as well as in those with MI with non-obstructive coronary arteries, referred to as MINOCA.30,67–69,71,72,74,77–83 The mechanisms and aetiologies of INOCA are multifactorial and heterogeneous, often overlapping and coexisting in various combinations in a subclinical fashion.13,14,30,66,67,72,77 Indeed, a substantial proportion of patients with INOCA differ in the underlying aetiology of myocardial ischaemia. If complicated with CMD, the patient outcomes are at stake with increased future adverse cardiac events.20,22–33 Thus, comprehensive and multidisciplinary assessment of coronary vasomotor reactivity should be adopted to endotype patients with INOCA based on the underlying mechanisms of coronary vasomotion abnormalities for better risk stratification and management.14,18,45 Coronary microvascular spasm should not be dismissed under the umbrella of normal coronary arteries.
Diagnosis of Coronary Microvascular Spasm
The COVADIS Group has proposed a consensus set of standardised diagnostic criteria for MVA composed of four essential elements: symptoms of myocardial ischaemia; absence of obstructive coronary artery disease on coronary angiography; objective evidence of myocardial ischaemia; and evidence of impaired coronary microvascular function.65 The documentation of coronary microvascular spasm is listed as one of the standard criteria for impaired coronary microvascular function, defined as the reproduction of symptoms, ischaemic ECG changes, but no epicardial spasm during acetylcholine provocation testing.65 The diagnostic value of these criteria has been demonstrated by recent clinical studies.14,30,72,76
In equivocal cases with non-diagnostic results for coronary microvascular spasm, such as reproduction of symptoms during the provocation test without ischaemic ECG changes and vice versa, myocardial ischaemia attributable to coronary microvascular spasm can be diagnosed using myocardial lactate extraction ratio or desaturation of coronary sinus blood.65,68–70,78,84,85 Lactate arises when the rate of glycolysis exceeds that of oxidation in the setting of anaerobic metabolism due to cellular hypoxia and thus transient metabolic alterations represented by myocardial lactate production serve as an objective marker of myocardial ischaemia during the spasm provocation testing.86 Briefly, blood samples are obtained simultaneously from the coronary sinus and left coronary artery during the spasm provocation testing.68,84 The net production of lactate across the coronary circulation can be calculated as (coronary sinus concentration – left coronary arterial concentration) divided by left coronary arterial concentration; a positive value indicates pathological production or release and a negative value physiological uptake or metabolism of lactate in the coronary circulation.68,84 Thrombolysis in MI (TIMI) frame count, a marker of coronary blood flow, during the spasm provocation testing may be an alternative indirect method to diagnose the occurrence of coronary microvascular spasm.78
Pathophysiology of Coronary Microvascular Dysfunction
Endothelial Modulation of Vascular Tone and Therapeutic Considerations
The endothelium, a mono-layer of endothelial cells lining the cardiovascular system, plays a pivotal role in modulating vascular tone by synthesising and liberating endothelium-derived relaxing factors (EDRFs), including vasodilator prostaglandins (e.g. prostacyclin), nitric oxide (NO), and endothelium-dependent hyperpolarisation (EDH) factors as well as endothelium-derived contracting factors (EDCFs) in response to shear stress and various agonists in vivo (Figure 2).41,44 EDH-mediated responses are observed in the presence of cyclooxygenase and NO synthase inhibitors and are accompanied with hyperpolarisation and relaxation of the underlying vascular smooth muscle cells (VSMC) and subsequent vasodilatation.41,44 Although the nature of EDH factors appears to vary depending on species and vascular beds, endothelium-derived hydrogen peroxide at physiological low concentrations plays a major role in EDH-mediated relaxations in the coronary circulation of humans and animals.87–91 In-depth information on other EDH factors is available elsewhere.41 These endothelium-derived mediators have a distinct vessel size-dependent role universally across a range of species from rodents to humans; NO predominantly mediates vasodilatation of relatively large, conduit vessels (e.g. epicardial coronary arteries), while EDH factors in small resistance vessels (e.g. coronary arterioles and microvessels) (Figure 2).41,44,92,93 Consequently, EDH-mediated relaxations are an important vasodilatory mechanism in coronary microcirculation, where coronary vascular resistance is predominantly determined. Coronary blood flow actually does not increase in response to intracoronary administration of nitroglycerin.94 EDH-mediated responses are enhanced in small resistance arteries through multiple mechanisms, including negative interactions between NO and several EDH factors.95,96
Endothelial dysfunction manifests itself as reduced production or action of EDRFs or increased responses of EDCFs.41,44 Along with coronary microvascular spasm, endothelial dysfunction contributes to the development of CMD.39–41 A large-scale cohort study (n=1,439) from the Mayo Clinic showed that as many as two-thirds of patients with INOCA had either endothelium-dependent or -independent CMD as a plausible aetiology of symptoms and signs of myocardial ischaemia, which was evaluated by invasive functional coronary angiography.13 Endothelium-dependent CMD is associated with coronary atherosclerosis and vulnerable plaque characteristics in patients with INOCA.94 Moreover, recent studies have revealed that CMD is a cardiac manifestation of the systemic small artery disease beyond the heart because of its concomitance with peripheral endothelial dysfunction.14,28,67 For example, both NO- and EDH-mediated digital vasodilatations in response to bradykinin are markedly impaired in patients with MVA.67
Contrary to a simple assumption that enhancing NO-mediated vasodilatation through supplemental NO could exert beneficial effects on patients with various cardiovascular diseases in whom the prevalence of CMD is not negligible, the effects of systemic and long-term administrations of nitrates were disappointingly unsuccessful or even harmful in patients with residual microvascular ischaemia despite successful PCI, vasospastic angina, and heart failure with preserved ejection fraction.97–100 It may be important to consider the blood vessel size-dependent contribution of NO and EDH factors in the treatment of CMD and to tailor the most appropriate therapy based on the underlying mechanisms of coronary vasomotion abnormalities (Figure 2).
Rho-kinase and Vasoconstrictive Mediators
Coronary vasoconstriction and vasospasm are the important determinants of supply ischaemia or primary angina, even in patients who have no obstructive stenosis or angiographically normal coronary arteries.44,46,101 The degree of coronary vasoconstriction is determined by the potency/efficacy of constrictor stimuli and the sensitivity/reactivity of blood vessels.46 Rho-kinase-induced myosin light chain phosphorylation with resultant VSMC hypercontraction is a major mechanism in the pathogenesis of coronary vasospasm (Figure 2).44,102–104 Intracoronary administration of fasudil, a selective Rho-kinase inhibitor, is effective for relieving refractory epicardial coronary spasm resistant to calcium-channel blockers or nitrates, coronary microvascular spasm, and the coronary slow flow phenomenon after PCI, although this drug has not yet been approved for clinical use in western countries.105–107 Moreover, Rho-kinase activity is elevated in patients with vasospastic angina who have high coronary microvascular resistance.30 Enhanced epicardial and coronary microvascular constriction/spasm is associated with increased production of vasoconstrictive mediators, including neuropeptide Y, endothelin-1, serotonin, and inflammatory responses as discussed below (Figure 2). 61,108–112 These vasoconstrictors have the potential to cause coronary artery constriction and myocardial ischaemia above the coronary autoregulation.113
Inflammation and Coronary Vasospasm
Chronic low-grade vascular inflammatory responses play important roles in the underlying mechanisms behind coronary vasospasm and CMD.61 Professor Maseri paved the way for the inflammatory hypothesis of atherosclerotic cardiovascular diseases by simply showing that patients with acute coronary syndromes who had elevated levels of C-reactive protein on admission were associated with worse outcomes.114 After a quarter century of research, this hypothesis was proved to be true by the results of the CANTOS study; treatment with canakinumab, a selective anti-interleukin-1β monoclonal antibody, significantly reduced the risk of recurrent cardiovascular events in patients with a history of MI who had a high-sensitive C-reactive protein level of 0.2 mg/dl or more as compared with placebo.115 Building on the inflammatory hypothesis, close relationships among inflammation, perivascular adipose tissue (PVAT), and vasa vasorum have emerged as key players in the pathogenesis of coronary artery spasm. A major inflammatory cytokine interleulin-1β promotes intimal thickening and coronary vasospastic responses to intracoronary serotonin or histamine via outside-to-inside signalling in pigs in vivo.116 Enhanced formation of adventitial vasa vasorum, which serves as a conduit for inflammatory mediators derived from the surrounding inflamed adipose tissue to the local coronary wall, is associated with coronary hyperconstriction via Rho-kinase activation in patients with vasospastic angina.117 Treatment with calcium-channel blockers attenuates inflammation of PVAT in the spastic coronary artery in patients with vasospastic angina in a reversible manner.118 Coronary vasospasm at epicardial and microvascular levels can be accompanied by active myocardial inflammation of cardiac sarcoidosis and immunosuppressive therapy in combination with calcium-channel blockers may be effective for alleviating the severity of coronary artery spasm in parallel with regression of myocardial inflammation of the disease.119
Conclusion
The epoch-making results of the two landmark clinical trials, the ORBITA and ISCHEMIA trials, for the management of stable coronary artery disease serve to recall the importance of coronary vasospasm and CMD beyond obstructive coronary artery disease.35,36,38,120 Professor Maseri was correct in his “search” for the origin of myocardial ischaemia in the absence of obstructive coronary arteries long before practice guidelines were published on the management of CCS including INOCA.1,2,18,45,63 He was also resourceful in his emphasis on that “elucidation of its mechanisms will lead to more appropriate therapy”.121 Cardiologists need to respond to the developing evidence irrespective of the performance measures we may adhere to in daily practice. Coronary vasomotion abnormalities will become increasingly important in the post-ISCHEMIA era. Further research is warranted to develop novel therapeutic strategies for coronary vasomotion abnormalities to improve the clinical outcomes of patients with the disease.