Cancer and cardiovascular disease are the two main causes of death worldwide in both men and women.1 One in 8.3 deaths was attributed to coronary heart disease (CHD) in women, whereas one in 31.5 deaths was attributed to breast cancer (BC) in 2017.2
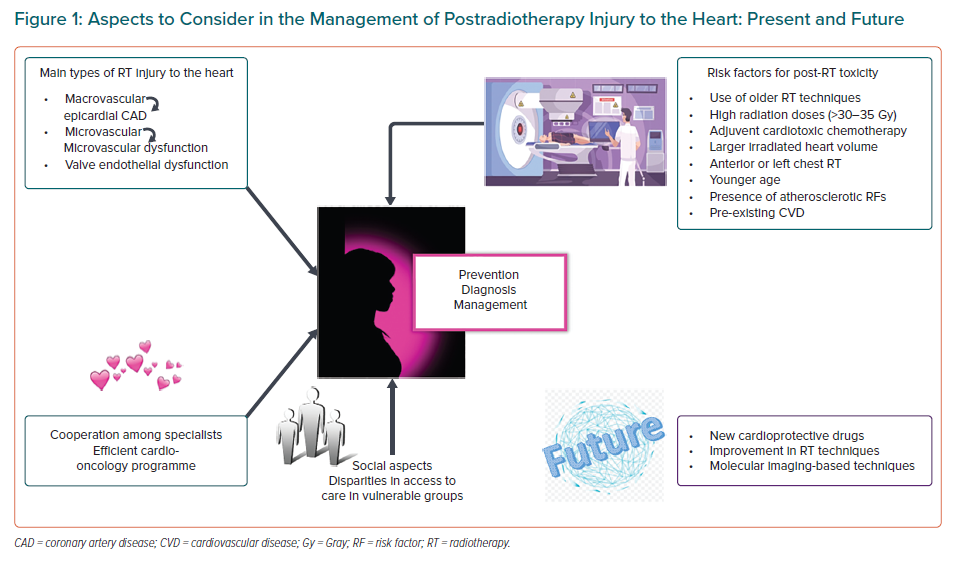
In the past decades, survival rate in cancer patients has substantially improved due to new treatments and developments in radiation therapy (RT). In women, BC is the leading cause of cancer death and thoracic RT is a main component of the treatment in many cases.3 In this review, the following aspects will be addressed: pathophysiology of postradiotherapy heart damage in women with BC; mechanisms, diagnosis and prevention/management of heart damage; and future areas of potential research for radiotherapy injury in women (Figure 1).
Radiotherapy
RT to the chest area is frequently part of the treatment for Hodgkin lymphoma and cancers of the lung, oesophagus or breast. Although RT reduces the risk of mortality for these cancers, it can also lead to increased non-cancer-related mortality, primarily due to cardiac causes. Major risk factors that increase the likelihood of cardiac toxicity include use of older RT techniques, high radiation dose (more than 30–35 Gy), adjuvant treatment with cardiotoxic chemotherapy (mainly anthracyclines), larger irradiated heart volume, anterior or left chest RT, younger age at treatment, the presence of atherosclerotic cardiovascular risk factors such as smoking, obesity/overweight, hypertension, hyperlipidaemia, diabetes and a sedentary lifestyle. Radiation can damage all cardiac structures.
For the purpose of this review, we will refer mainly to RT to treat BC in women. Darby et al. reported a linear increase in relative risk of major coronary events of 7.4% per Gy increase in mean heart dose, independently of the presence of cardiac risk factors, without any threshold safe dose in which no events were observed.4 This increased risk was observed shortly after exposure and continued for at least 20 years. However, this study was limited by use of older treatment techniques. CT-based planning was not performed and mean heart doses were estimated from 2D techniques.
In a systematic review of individual patient data published from 2010 to 2015, including more than 40,000 patients with a median follow-up of 10 years, Taylor et al. demonstrated an increased risk of cardiac mortality with an increased risk ratio of 1.3 (95% CI [1.15–1.46]) and a 0.04 excess rate ratio of cardiac mortality per Gy of whole-heart dose. Previous diagnoses of ischaemic heart disease and smoking were confounding factors for risk of cardiac death.5
Thanks to new techniques where a more limited area receives radiation therapy (RT), exposure of the heart has reduced substantially over the past few decades. At present, the mean heart dose from BC RT worldwide may be around 5 Gy in left-sided RT and 3 Gy in right-sided, but with significant variations according to country, patient anatomy and technique.6
In a meta-analysis including 39 studies involving 1,191,371 women with BC treated with RT, Cheng et al. found that those who received left-sided RT, as compared with those receiving right-sided RT, experienced increased risks of developing CHD (RR 1.29; 95% CI [1.13–1.48]), cardiac death (RR 1.22; 95% CI [1.08–1.37]) and death from any cause (RR 1.05; 95% CI [1.01–1.10]).7 Of note, the risk started to increase within the first decade post-RT for CHD and from the second decade for cardiac mortality.
Mechanisms of Heart Damage Caused by Radiotherapy
Exposure to chest radiation causes three main types of injuries to the cardiac structures:
- Macrovascular injury that accelerates age-related atherosclerosis and causes coronary artery disease (CAD) decades after RT;
- Microvascular injury that reduces myocardial capillary density, months post-RT. This originates a reduction of collateral flow/vascular reserve with resulting myocardial ischaemia, as well as an increased capillary permeability of the pericardium with resulting thickening and adhesions; and
- Valve endothelial injury and dysfunction causing valve disease.8
All of these three physiopathological mechanisms are worsened with concomitant cardiotoxic chemotherapy and can lead to systolic and diastolic dysfunction and clinical heart failure (HF).
Although the underlying mechanism of the gradual progression from the asymptomatic early stage to CHD is complex and not fully understood, it is believed that this damage is associated with endothelial cell injury, inflammatory reaction with release of pro-inflammatory cytokines, oxidative stress, mitochondria and endoplasmic reticulum injury, calcium overload and microRNAs.7,9,10 When any tissue is irradiated, the cells comprising the tissue are damaged primarily by the generation of free radicals, mainly the hydroxyl radical. In addition to releasing pro-inflammatory cytokines, free radicals react with DNA and cause strand disruption, inhibiting suitable replication and protein synthesis. Despite the relative resistance of cardiomyocytes to radiation, doses used at present can cause damage to cardiac structures. It is proposed that early injury is mainly caused by acute and chronic inflammatory changes, while late damage is partly caused by oxidative stress and inflammation.11
How to Diagnose Heart Damage Postradiotherapy
When clinicians have to treat and follow women with thoracic cancer, such as BC, who have had RT, the first thing to consider is the probability of cardiac damage months to years after RT, even with new planning and radiation techniques.
Another important consideration for diagnosis and management of postradiotherapy damage is the impact of early-life cardiovascular (CV) risk factors on cancer survivorship. As survival rates from cancer increase, CV disease (CVD) in survivors has become increasingly prevalent. Thus, under-treatment and under-recognition of CVD in younger patients with CV risk factors should be taken into account as a cause of higher CV mortality in these patients.
Carlson et al. studied 972 women without pre-existing CVD who received RT for BC in the WECARE study.12 They found that women treated with left-sided RT had a greater than twofold risk (HR 2.5) for development of CAD compared to women treated with right-sided RT. The additive risk of left-sided RT appears particularly pronounced in younger patients – those aged 25–39 years receiving left-sided RT had a 27.5-year CAD risk of 5.9% versus 0% in those receiving right-sided RT. This shows the need for prolonged surveillance for CAD in younger cancer survivors. Given the latency between radiation exposure and the development of cardiovascular events, it is important that young women who have received left breast RT be considered at higher risk over their lifetime. Reversible or modifiable cardiac risk factors worsen the cardiovascular late effects of RT and should be aggressively controlled in this population.
From a public health perspective, the prevention of the development of risk factors in the first place (primordial prevention) is mandatory. Once risk factors have developed, restoration of low risk for CVD and cancer becomes very unlikely.
There are currently no worldwide-accepted screening guidelines for radiation-induced cardiac toxicity. Nevertheless, considering the known association of the already mentioned risk factors, it is reasonable to suggest that the identification of these patients and close follow-up could facilitate early diagnosis and treatment of any cardiac side effects. Cardiac imaging, including cardiac magnetic resonance (CMR), CT, echocardiography and myocardial perfusion imaging (MPI), provide the highest diagnostic yield. Undoubtedly, echocardiography is the first line to diagnose cardiac damage due to its availability, but CMR is a better option to understand the pathology and severity of radiation-induced damage.
Clinical Manifestations of Radiation T-induced Cardiac Damage8,13,14
The main clinical manifestations of RT-induced cardiac damage are coronary artery disease, pericardial involvement, cardiomyopathy, valve disease and conduction system abnormalities.8,13,14
Coronary Artery Disease
Retrospective cohort studies have shown that between a few months and more than 25 years after thoracic RT, 5–10% of the patients developed moderate-to-severe CAD.15,16
CAD can appear early or a long time after RT. Acute CAD does not have immediate apparent effects, although 47% of patients can show perfusion defects in MPI and regional wall motion abnormalities 6 months after RT. As in atherosclerotic CAD, electrocardiography and serum cardiac biomarkers, such as troponin and creatinine kinase-MB, are helpful when acute coronary syndrome (ACS) is suspected.
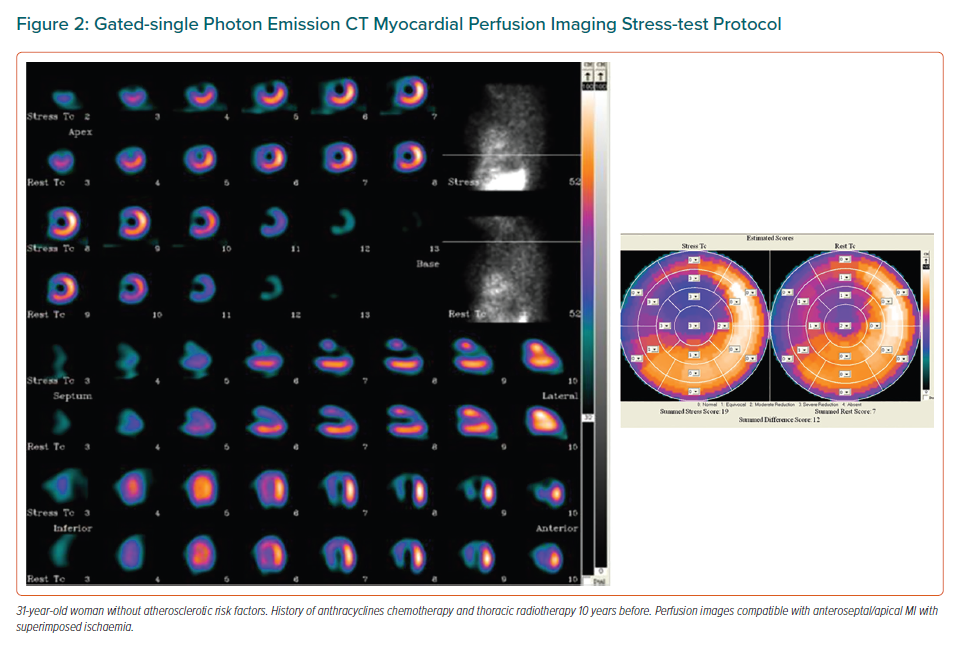
At long term, although accelerated CAD can appear in younger people, concomitant atherosclerotic risk factors enhance the development of CAD. Detection of ischaemia with imaging tests is indicated in these patients. 99mTc-tetrofosmin single photon emission CT (SPECT) MPI has been validated for this purpose (Figure 2).17
Stress echocardiography, either with dobutamine or physical stress and CMR, may also help to detect ischaemia. Following an anatomical approach, coronary CT angiography (CCTA) with or without coronary artery calcium (CAC) scoring is another technique to diagnose CAD.18
Groarke et al. studied 35 patients (71% women, mean = 66 ± 11 years) referred clinically for PET/CT MPI at a median (interquartile range [IQR]) interval of 4.3 (2.1–9.7) years following RT for cancer disease. The median (IQR) of mean cardiac radiation doses was 12.0 (1.2–24.2) Gy. They found significant inverse correlations between mean radiation dose and global myocardial flow reserve (MFR) (MFRGlobal) and MFR in the left anterior descending artery territory (MFRLAD): Pearson’s correlation coefficient 2.37 (p=0.03) and 2.38 (p=0.03), respectively.19
Tagami et al. screened 6,593 patients with a history of non-metastatic BC treated with RT using CCTA. Patients were matched for age and risk factors. Left BC had higher rates of CAD compared with right BC patients – LAD coronary artery 76% versus 31% (p<0.001), left circumflex 33% versus 6.7% (p=0.004), and right coronary artery 37% versus 13% (p=0.018). Mean LAD radiation dose and mean heart dose strongly correlated with CAD with a 21% higher incidence of disease in the LAD.20
In addition to the proliferation of the intima layer common to both RT-induced and typical coronary atherosclerosis, CAD induced by RT causes thinning of the media layer and extensive fibrosis of the adventitia. It appears in younger patients and typically affects coronary ostia and proximal segments. The distal right coronary artery is only involved if there is cancer of the lower oesophagus.
Lai et al. studied 94 patients with non-contrast CT (NCCT) of the thorax before and after adjuvant RT, including both left- and right-side BC. Their CAC burden was compared with healthy controls from the MESA cohort. The progression of the CAC burden was manifested by the increment of CAC percentiles (%CACinc = CACpercentile after RT − CACpercentile before RT). Ninety-two out of 94 patients had zero CAC percentiles before RT and 68 patients were still zero after receiving adjuvant RT. Twenty-six patients had positive %CACinc after adjuvant RT, 19 left-side and 7 right-side BC patients. The mean values of %CACinc from the first to second NCCT were 18.4%, 25.3%, 10.2% and 2.7% in total, left-side, right-side BC patients, and non-BC women, respectively.21
Microvascular dysfunction rather than epicardial CAD may cause non-reversible defects on MPI or perfusion defects that do not correspond to coronary artery territories. Myocardial blood flow reserve assessed by PET allows this microvascular dysfunction to be diagnosed.
Myocardial flow reserve, defined as the ratio of myocardial blood flow at peak stress to that at rest, is a measure of large and small coronary vessel function that can be non-invasively assessed using PET. Impaired MFR predicts major adverse cardiovascular events in patients with and without flow-limiting CAD.22,23
Myocardial flow reserve, which is able to measure the effects of epicardial CAD, diffuse atherosclerosis, vessel remodelling, and microvascular dysfunction, represents a useful indicator of radiation-induced coronary injury, given the diffuse nature of radiation damage.
Radiation-induced endothelial dysfunction of the microcirculation in addition to radiation injury to the cardiac sympathetic nerve fibres, as well as chronic, subclinical, low-grade inflammation may explain the association between radiation dose and impaired MFR.
Measurement of MFR with CMR can also help to identify microvascular dysfunction in postradiotherapy patients.24–26 For instance, in 46 patients with typical angina, no obstructive CAD and risk factors for microvascular disease, Zorah et al. showed that by quantitative CMR perfusion imaging there is a reduction in absolute stress myocardial blood flow and an overall reduction in MFR when compared with normal controls, suggesting that microvascular disease may be a possible cause of symptoms.25 This option may also be considered in postradiotherapy patients.
Pericardial Involvement
Pericardial involvement ranges from 70% to 90% among patients who received more than 35 Gy and can appear as fibrous thickening, pericardial effusion with the potential for tamponade, fibrinous pericardial adhesions, acute pericarditis, pericardial constriction and obliterative pericardial fibrosis.27 The onset of symptoms of acute pericarditis may occur immediately after radiotherapy to 2 years later and includes chest pain and a friction rub, generally with recovery. Chronic pericarditis typically presents 10 or more years after radiotherapy. Diagnosis is similar to other causes of pericardial damage.
Cardiomyopathy
Radiotherapy can cause cardiomyopathy due to direct injury and myocardial fibrosis or ischaemia. A case-control study on women with BC who received contemporary conformal RT with a dose less than 5 Gy showed that HF with preserved EF (HFpEF) developed at an average of 5.8 years post-RT. The risk of developing HFpEF was 16 times greater than controls, was more frequent in patients with ischaemic heart disease or AF and increased with RT dose.28
Valve Disease
Up to 81% of patients with RT-induced heart damage develop valvular disease. Clinically significant disease appears at doses above 30 Gy. It can lead to either stenosis or regurgitation. Thickening of the aorto-mitral curtain is the key feature of RT-induced valvular disease, with a higher risk for left-sided valves, with the aortic valve being the most commonly affected, likely due to the high-pressure transvalvular gradient coupled with proximity to the RT field.27,29 In the mitral valve, RT injury typically spares the leaflet tips and valve commissure.
Conduction System Abnormalities
The direct effect of radiation on the conducting fibres or the indirect mechanism due to fibrosis of the myocardium surrounding the conduction system is responsible for conduction system abnormalities that appear in approximately 5% of cases, such as atrioventricular block, sinus node syndrome, QTc prolongation, supraventricular arrhythmia and ventricular tachycardia.30 These abnormalities often occur within 2 months after the end of RT, and 70% can return to normal after 6 months after the end of RT.31
How Post-radiotherapy Patients Should be Followed-up
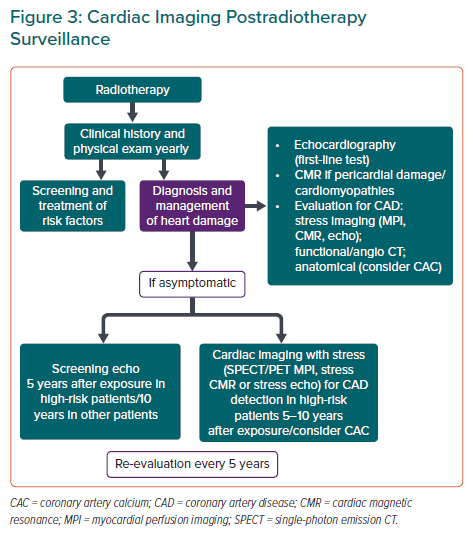
According to the expert consensus for multimodality imaging evaluation of CV complications of RT in adults, evaluation based on clinical picture, electrocardiographic and echocardiographic surveillance should be implemented in patients 5 years after RT treatment in high-risk patients and 10 years after in all other patients.8,32 Further reassessment should be performed every 5 years. Even asymptomatic, high-risk patients should be referred for functional non-invasive stress tests within 5–10 years of completing RT (Figure 3). Of note, pregnant women and those who are planning pregnancy should be carefully monitored as gestation may unmask subclinical cardiotoxicity.
Bearing in mind that non-obstructive CAD often goes undetected using functional stress testing if there is no demonstrable ischaemia, a valuable option to consider is the assessment of CAC even in CTs performed for RT planning. The identification of CAD will allow the implementation of aggressive risk factor control, which will be an important step to reduce post-RT heart damage.
Prevention and Management
Prevention, as in the vast majority of diseases, is the main line of action to reduce RT damage to cancer patients. In this sense, it is necessary to consider new techniques to minimise the radiation dose to the heart, in addition to ensuring there is adequate risk stratification for patients with a higher risk of radiotoxicity in whom dose control measures are mandatory.32–34 A comprehensive evaluation prior to the initiation of RT should be done for all patients, including a medical history, a physical examination and a baseline echocardiogram with emphasis on CV assessment of pre-existing risk factors and CV disease before, during and after treatment.
The American Society for Radiation Oncology guidelines for whole breast RT recommend minimising the cardiac dose as much as possible by excluding the heart from the primary treatment fields and using other techniques.35 The expert panel pointed out that mean heart doses under 1.0–2.0 Gy (left-sided) and under 1.0 Gy (right-sided) are usually achievable when regional lymph nodes are excluded, as well as a mean heart dose of <4 Gy is considered acceptable when the regional lymph nodes are included. 3D imaging with CT at the time of treatment planning enhanced visualisation of the entire heart, allowing the exclusion of the heart and the chain of internal mammary lymph nodes from the field. This, combined with improved dose/therapy calculations using computerised modelling software, allow radiotherapists to measure the actual dose being received by the heart.
On the other hand, the use of radiation techniques such as deep inspiration breath hold and prone positioning allows the increase of distance between the heart and the irradiated chest wall or breast, resulting in significant cardiac dose reductions.36 Other technical approaches to minimise heart dose during RT for BC include optimisation of beam angles, use of multileaf collimator shielding, intensity-modulated radiotherapy, proton therapy and partial breast irradiation.37–39 Every possible effort should be made to reduce the dose to the heart during RT but without compromising the coverage of the target, thus maintaining the beneficial effect of the treatment.
The use of CV disease prophylactics, such as angiotensin-converting enzyme inhibitors, ß-blockers and statin therapy, as cardioprotective agents, has been recommended by the European Society for Medical Oncology in patients with pre-existing CV disease who are receiving oncological therapy.40 However, the 2022 European Society of Cardiology (ESC) guidelines on cardio-oncology state there are no proven medical therapies to prevent RT-induced CV toxicity.41 On the other hand, they also recommend a tight control of CV risk factors considering that one component of RT-induced CV toxicity is accelerating pre-existing CAD. Thus, the 2022 ESC guidelines recommend a baseline CV risk assessment and estimation of 10-year fatal and non-fatal CV disease risk using SCORE2 or SCORE2-OP in patients who are due to have RT that would affect the heart (recommendation class I, level of evidence B).41
Management
Coronary Artery Disease
The 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes made two recommendations for the management of patients with active cancer and chronic coronary syndromes (CCS):
- Treatment decisions should be based on life expectancy, additional comorbidities such as thrombocytopaenia, increased thrombosis propensity, and potential interactions between drugs used in CCS management and antineoplastic agents (recommendation class I, level of evidence C);
- If revascularisation is indicated in highly symptomatic patients with active cancer and increased frailty, the least invasive procedure is recommended (recommendation class I, level of evidence C).42
It is important to consider that the internal mammary nodes are often included in regional nodal radiation in patients with BC, with the internal mammary vessels (left internal mammary artery in left-sided patients) in the first three intercostal spaces considered as a surrogate target.43
The 2022 ESC guidelines on cardio-oncology recommend the preoperative assessment of internal mammary artery viability, venous access and sternal wound healing in cancer survivors with RT-induced symptomatic CAD where coronary artery bypass graft (CABG) is considered (recommendation class I, level of evidence C).41
Percutaneous coronary intervention (PCI) with drug-eluting stents may be considered over CABG in cancer survivors with RT-induced severe left main or three-vessel disease, with a high SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) score (>22), in whom the planned PCI is technically feasible given the increased complications associated with CABG after mediastinal RT (recommendation class IIb, level of evidence B).41
In asymptomatic radiation-induced CAD detected during surveillance of cancer survivors, non-invasive stress testing is recommended in asymptomatic cancer survivors with new moderate or severe radiation-induced CAD detected on CCTA to guide ischaemia-directed management (recommendation class I, level of evidence C).41
Valve Disease
Valve replacement is the pillar of treatment for severe valve disease. However, the perioperative (30-day) mortality rate is 12% and the 5-year survival rate is 66%.44,45 Thus, in general, transcatheter valve replacement is preferred in patients with higher radiation doses to the mediastinum, with prior cardiac surgery with extensive mediastinal fibrosis or those who have significant surgical risks (that can contraindicate surgery).
The 2022 ESC Guidelines on cardio-oncology recommend transcatheter aortic valve implantation (TAVI) for patients with symptomatic severe aortic stenosis caused by radiation who are at intermediate surgical risk (recommendation class IIa, level of evidence B). It is emphasised that a multidisciplinary team approach should be followed to discuss and define the surgical risk in cancer survivors with severe valvular heart disease if case surgery is the chosen option.41
Pericardial Complications
According to the 2022 ESC guidelines on cardio-oncology, patients with acute pericarditis during RT to an area that includes the heart are at higher risk of developing chronic constrictive pericarditis. Therefore echocardiography every 5 years may be considered as a recommendation (class IIb, level of evidence C).41
Percutaneous balloon pericardiotomy or pericardial window creation should be used in selected cases for large or growing chronic effusions if haemodynamic compromise appears.
Heart Failure
In patients with prior chest RT and HFpEF, consideration of both restrictive cardiomyopathy and constrictive pericarditis is recommended, with management of volume and blood pressure.32 Pericardiectomy can be considered in those with prior chest RT and confirmed constrictive pericarditis without response to medical management.
Disparities in Access to Good Care and Outcomes in Under-represented Patient Populations and Socioeconomic-related Disparities
Cancer and CVD are the two main causes of morbidity and mortality worldwide. Moreover, there is a growing population of patients who have both cancer and CVD, with a significantly higher economic burden to face. Socioeconomic differences and inequality play an important role in the incidence, treatments and outcomes of both cancer and CVD.46,47
Social determinants, such as economic stability, educational access and quality, healthcare access, living environment, structural racism, lack of insurance, food insecurity and social and community context, influence different aspects of health, functional behaviour, quality of life and disease outcomes. For instance, despite improvements in preventive and treatment strategies for CVD, an increase in the prevalence of cardiometabolic risk factors has contributed to a rise in premature and overall cardiometabolic mortality in the US, being particularly significant among black people living in poor neighbourhoods in south-eastern states.48,49 On the other hand, although better cancer therapeutics have improved overall outcomes for patients with many types of cancers, cancer-related mortality remains prominent in rural areas, with high urban-rural differences.50
Furthermore, screening for breast, cervical and colorectal cancers is lower among Hispanic, Asian, Native American and Alaskan people compared with white and black people, making them vulnerable to poor cancer-related outcomes.51
It is also important to consider the situation of younger people. Although reported mortality due to cancer and CVD is higher in individuals >45 years of age, the impact on mortality of increasing levels of social vulnerability has been more pronounced in those <45 years of age. Possible reasons are an increase in the prevalence of traditional CVD risk factors in younger adults, rising unemployment and poverty, lack of affordable health insurance and limited access to healthcare, higher prevalence of risky behaviours (tobacco, alcohol and drug use) being more evident in people living in the most vulnerable areas.52,53
Regarding sex, Khan et al. showed a rise in mortality related to CVD and cancer in young and middle-aged women in the US.54 In the ARIC study, it has been reported there is a higher incidence of traditional risk factors – hypertension, diabetes, and stroke – in women.55 In addition, women are more affected than men by psychosocial factors, such as stress, social isolation and depression, with a negative influence on mortality.56
This situation is even worse in low- and middle-income countries where fewer economic resources are allocated to public health and fewer vulnerable and under-represented population groups receive appropriate care.
Actions to improve healthcare outcomes in these patients, include investing more in healthcare infrastructure in socially vulnerable areas, educating healthcare providers about the impact of social determinants on chronic diseases outcomes, considering the patient as a bio-psycho-social being and building more integrated healthcare delivery systems where access to adequate care can be guaranteed for all patients.
Building a Good Cardio-Oncology Programme Through Collaboration Among Specialists
The ultimate goal of cardio-oncology is to allow patients with cancer to receive the best possible cancer treatments safely, minimising cardiotoxicity due to cancer drugs and radiotherapy.57 A guiding principle of cardio-oncology is the integration of clinical disciplines. Thus, cardio-oncology providers must have knowledge of the broad scope of cardiology, oncology, and haematology management, and a good collaboration among cardiologists, oncologists, haematologists, radiation oncologists and radiologists needs to be established to build an efficient cardio-oncology programme.
In 2014, the American College of Cardiology (ACC) National Cardio-Oncology Survey identified multiple factors that have traditionally acted as barriers to developing cardio-oncology programmes, including limited resources and interest, lack of infrastructure and lack of educational opportunities.58 This situation, including the lack of cooperation among different related specialists, is an international phenomenon that needs urgent attention.
The ESC Guidelines on cardio-oncology consider that an important gap to address is the role of cardio-oncology services and cardio-oncology care networks, including the following aspects:
- Robust evidence on the impact of dedicated cardio-oncology and rehabilitation programmes on the prognosis of patients with cancer and cancer survivors;
- Specification of roles of different healthcare professionals (including nurses and pharmacists) in cardio-oncology teams;
- Cardio-oncology care networks to improve the management of patients with cancer and to discuss difficult cases;
- Cardio-oncology team support and involvement in oncology trials design; and
- Greater understanding of how to engage patients with cancer in their own CV care.41
Future Areas of Potential Research for Cardiotoxicity in Women
At present, more clinical trials to assess a better way to manage cardiotoxicity, including post-RT heart damage, are needed. In addition to that, animal experiments show that traditional drugs used for CVD, such as anti-platelet drugs and statins, have limited preventive effects on radiation-induced CVD.59 Therefore, there is an urgent need to identify new treatment modalities, including new treatments for prevention based on physiopathology of heart damage, as well as new developments in RT techniques that reduce heart dose while preserving the efficacy of the anti-cancer treatment.
In this line, molecular imaging-based techniques may constitute a strong option to understand the precise pathophysiology of the disease, contributing to developing targeted therapies and preventing organ dysfunction.
Another topic to consider is the use of cardiac biomarkers for prediction of cancer-therapy-related cardiovascular toxicity, including N-terminal pro B-type natriuretic peptide and troponins.60 Although heart damage due to several oncological treatments has been linked to these biomarkers, its real value is still unclear. Thus, it is another gap in the knowledge that needs to be addressed.
It is hypothesised that at present, atherosclerotic CVD may underestimate the risk of cardiovascular events in cancer patients. Thus, an optimised risk score, considering sex and potentially including CAC, is necessary for better management.
Conclusion
In women, BC treatments, including anthracyclines, HER2 antagonists and chest irradiation, are associated with significant cardiotoxicity. To achieve the best outcomes in these patients, it is crucial to optimise treatment to balance anti-cancer efficacy and cardiovascular safety. The identification of high-risk patients, aggressive management of their underlying cardiovascular risk factors, use of available cardioprotective strategies and periodic assessment of ventricular function before and after therapy are recommended.
Care of these patients should involve a multidisciplinary and collaborative approach, on an individual basis, including oncologists, haematologists, radiation oncologists, radiologists and cardiologists. The emergent field of cardio-oncology represents a good option to achieve the objective of reducing the risk of CVD following a cancer diagnosis.