Despite improvements in the management of coronary artery disease over the last decades, ischaemic heart disease remains the leading cause of mortality worldwide and the leading cause of premature death.1,2 Patients with chronic coronary syndromes (CCS) encompass a heterogeneous population, including those with demonstrable coronary artery disease by anatomical testing or evidence of ischaemia, prior MI and prior revascularisation. Most patients with CCS are free of angina, but some may have demonstrable ischaemia (silent ischaemia).3
Antiplatelet therapy, particularly aspirin, remains the cornerstone of the management of CCS for the prevention of atherothrombotic events.4–6 Additional therapies to improve platelet inhibition and reduce ischaemic events have been developed, but are often associated with an increase in bleeding events. P2Y12 receptor inhibition by clopidogrel alone as a substitute for aspirin failed to improve ischaemic outcomes in the coronary artery disease population, and the combination of clopidogrel and aspirin increased bleeding.7,8 New P2Y12 inhibitors, such as prasugrel and ticagrelor, reduced the risk of major cardiovascular outcomes when used for over 1 year after MI, but also increased the risk of bleeding.9,10 Ongoing trials are evaluating which patients with CCS may benefit most from these new P2Y12 inhibitors.11,12
Vitamin K antagonists (VKAs) have been used for many decades in patients with coronary syndromes for the prevention of atherothrombotic events, showing effectiveness in reducing the recurrence of major cardiovascular outcomes.13,14 However, increased bleeding related to a narrow therapeutic index requiring long-term careful monitoring and dose adjustment has resulted in VKAs being replaced by aspirin.15
Direct-acting oral anticoagulants (DOACs) have a favourable benefit/risk ratio (i.e. ischaemia/bleeding ratio) and easier use than VKAs, which could impact on the management of CCS, in the same way they have transformed the management of non-valvular AF (NVAF) and venous thromboembolism. To date, few studies have evaluated DOACs in this setting. Initial studies have focused on patients receiving DOACs for NVAF undergoing acute or elective percutaneous coronary intervention (PCI) who additionally required dual antiplatelet therapy (DAPT).
Direct-acting Oral Anticoagulants in Non-valvular AF and Percutaneous Coronary Intervention
Almost 10% of patients with CCS also have AF.16,17 Of those with AF, 25–35% also have a coronary syndrome.18–20 PCI is commonly needed in patients with NVAF treated with DOACs. The management of anticoagulant and antiplatelet therapies may be challenging. Several studies have evaluated this scenario.
Rivaroxaban and the PIONEER AF-PCI Trial
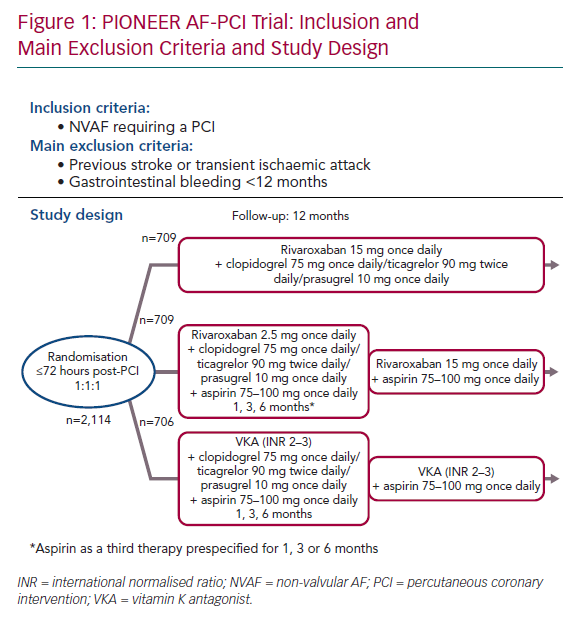
The Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Patients With AF Who Undergo PCI (PIONEER AF-PCI) was the first randomised study to evaluate patients treated with either rivaroxaban or warfarin for NVAF who would also need DAPT after PCI.21 The primary outcome was safety (major bleeds, minor bleeds or bleeding requiring medical attention) and the secondary outcome was efficacy (composite of cardiovascular death, MI or stroke). Between 2013 and 2015, 2,124 patients with NVAF who had undergone PCI with stent placement were recruited. They were at a low thromboembolic risk (average CHA2DS2-VASc score 1.6) and without any prior stroke or transient ischaemic attack (TIA) when enrolled. Almost half were known to have CCS. This open-label trial randomised patients in a 1:1:1 ratio to receive:
- Rivaroxaban 15 mg once daily plus single antiplatelet therapy (SAPT) with a P2Y12 inhibitor (clopidogrel 75 mg once daily, ticagrelor 90 mg twice daily, or prasugrel 10 mg once daily) for 12 months.
- Rivaroxaban 2.5 mg twice daily, with stratification to a prespecified duration of DAPT (for 1, 6 or 12 months) including low-dose aspirin (75–100 mg once daily) plus a P2Y12 inhibitor (clopidogrel 75 mg once daily, ticagrelor 90 mg twice daily, or prasugrel 10 mg once daily). After stopping aspirin, rivaroxaban was increased to 15 mg once daily (or 10 mg in the event of renal insufficiency) and the P2Y12 inhibitor was continued at the same dose.
- VKA as the control group, with stratification to a prespecified duration of DAPT (given by each investigator; 1, 6 or 12 months), including low-dose aspirin (75–100 mg once daily) plus a P2Y12 inhibitor (clopidogrel 75 mg once daily, ticagrelor 90 mg twice daily or prasugrel 10 mg once daily).
Randomisation was done up to 72 hours after the PCI, with conventional DAPT (including loading doses) up to 72 hours. For each group, clopidogrel was the most common P2Y12 inhibitor, used in >93% of cases (Figure 1).
At 12 months, the intention-to-treat analysis showed a significant reduction in bleeding for both rivaroxaban regimens compared with the VKA group. The HR for the primary safety composite outcome (including major bleeding, minor bleeding or bleeding requiring medical attention) was lower in the rivaroxaban 15 mg once daily regimen compared with the VKA regimen (HR 0.59; 95% CI [0.47–0.76]; p<0.001).22 The HR for the primary bleeding outcome was also lower in the rivaroxaban 2.5 mg twice daily group compared to the VKA regimen (HR 0.63; 95% CI [0.50–0.80]; p<0.001).23
There was no significant difference in the secondary efficacy outcome between the arms and no more in-stent thrombosis in the two rivaroxaban regimens compared with the VKA regimen.23 Despite being an exploratory and underpowered study, the results of PIONEER-AF suggested an equivalence of rivaroxaban and VKA regimens.
Dabigatran and the RE-DUAL PCI Trial
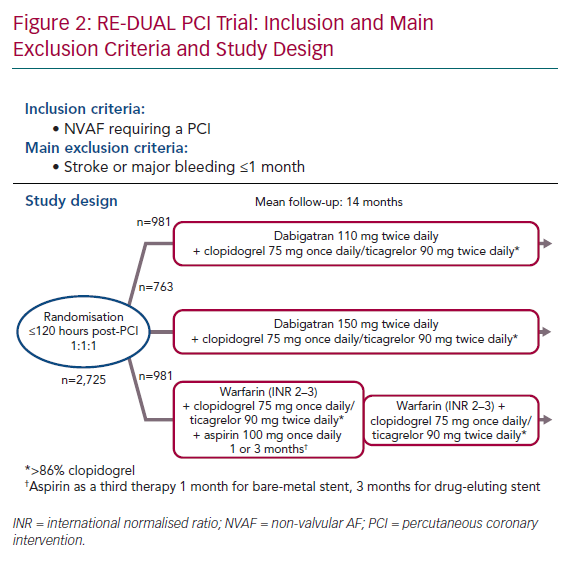
The non-inferiority Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF that Undergo a PCI With Stenting (RE-DUAL PCI) trial evaluated dabigatran in patients requiring oral anticoagulation for NVAF and DAPT for a PCI with stent insertion.24 In contrast to PIONEER AF-PCI, patients with stroke or TIA over 1 month previously were randomised. They were at a higher thromboembolic risk with a mean CHA2DS2-VASc score of 3.5. Between 2014 and 2016, 2,725 patients were enrolled. Nearly half were patients with CCS. This open-label trial randomised subjects to one of three regimens in a 1:1:1 ratio to receive:
- Dabigatran 110 mg twice daily plus SAPT with either clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily for the entire follow-up.
- Dabigatran 150 mg twice daily plus SAPT with either clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily, for the entire follow-up.
- Warfarin, plus DAPT with aspirin <100 mg once daily and either clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily. The duration of aspirin was 1 month for bare-metal stents and 3 months for drug-eluting stents.
Randomisation was done up to 120 hours after the PCI, with conventional DAPT up to 120 hours after the index PCI. Clopidogrel was the most common P2Y12 inhibitor, used in almost 90% of cases. No prasugrel was used in this trial (Figure 2). Mean follow-up was 14 months. The primary composite endpoint was the occurrence of major bleeding or clinically relevant non-major bleeding.25 The intention-to-treat analysis showed a significant reduction of bleeding in both dabigatran arms compared with warfarin. The HR for the primary bleeding outcome was lower in the 110 mg dabigatran + DAPT group than in the group receiving triple therapy with warfarin (HR 0.52; 95% CI [0.42–0.63]; p<0.001 for non-inferiority). Similarly it was lower in the dabigatran 150 mg + DAPT group (HR 0.72; 95% CI [0.58–0.88]; p<0.001 for non-inferiority) than in the group receiving triple therapy with warfarin. These results were consistent across several bleeding definitions.26 The composite efficacy endpoint of thromboembolic events (MI, stroke or systemic embolism), death, or unplanned revascularisation was similar between the treatment arms (HR 1.04; 95% CI [0.84–1.29]; p=0.005 for non-inferiority).26
RE-DUAL PCI confirmed that dabigatran was safer than warfarin regarding the occurrence of bleeding. Despite this trial also being underpowered to completely rule out an increase of ischaemic events in each dabigatran regimen – for that purpose 8,500 patients would have needed to be enrolled as initially designed – it strongly suggested that DOAC strategies are as effective as VKA strategies.
Apixaban and the AUGUSTUS Trial
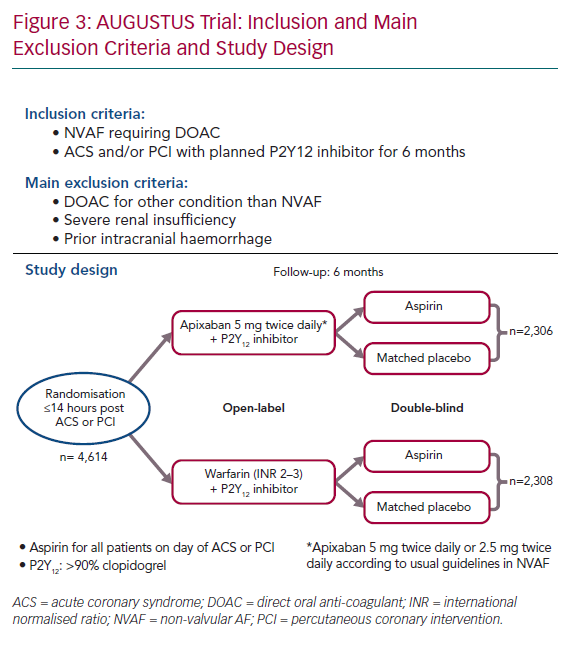
The Apixaban Versus Warfarin in Patients with AF and Acute Coronary Syndrome or PCI (AUGUSTUS) trial, enrolled >4,600 patients with NVAF (mean CHA2DS2-VASc score 3.9) and without severe renal insufficiency within 14 days after an acute coronary syndrome and/or a PCI.27 They were randomised using a two-by-two factorial design to receive: apixaban (with usual doses) versus warfarin (open label) and aspirin 81 mg versus placebo (double blind) for 6 months (Figure 3). At 6 months, patients received antiplatelet and anticoagulant therapy according to the local standard of care. Clopidogrel was the main P2Y12 inhibitor used (92.6%). The median time from the index event (acute coronary syndrome or PCI) to randomisation was 6 days (interquartile range 3–10) while all patients received aspirin. A total of 37.3% had acute coronary syndrome and underwent PCI, 23.9% had medically managed acute coronary syndrome and 38.8% underwent elective PCI.
At 6 months, the primary safety outcome, including major and clinically relevant bleeding, was lower in the apixaban group versus warfarin: 241 (10.5%) versus 332 (14.7%), respectively (HR 0.69; 95% CI [0.58–0.81]; p<0.001 for non-inferiority and superiority). The incidence of bleeding was higher in the aspirin arm versus placebo (HR 1.89; 95% CI [1.59–2.24]; p<0.001). No significant interaction was observed between the two randomisation factors.
The secondary outcome of a composite of all-cause death or all-cause hospitalisation was lower in the apixaban group versus warfarin: 541 (23.5%) versus 632 (27.4%), respectively (HR 0.83; 95% CI [0.74–0.93]; p=0.002). This was driven by a lower incidence of hospitalisation and stroke. This secondary outcome was similar in the aspirin versus placebo arm (HR 1.08; 95% CI [0.96–1.21]). There was no interaction between the two randomisation factors. All-cause death, MI and urgent PCI were similar between groups.
Thus, the AUGUSTUS trial further supported the safety of DOAC compared with warfarin in patients with NVAF requiring PCI and the safety of adjunctive SAPT with a P2Y12 receptor antagonist over DAPT with aspirin. While this trial confirmed the beneficial effect of apixaban on stroke, it was insufficiently powered to demonstrate a different effect on coronary outcomes (recurrent MI, stent thrombosis and urgent PCI).
Consequently, when concerns about coronary ischaemic risk prevail over concerns about bleeding risk, 1–6 months of triple therapy (oral anticoagulant + aspirin + clopidogrel) followed by dual therapy (oral anticoagulant + clopidogrel) are recommended to cover the period when the risk of stent thrombosis is presumed to exceed the risk of bleeding. When concerns about bleeding risk prevail over concerns about coronary ischaemic risk, a limited triple therapy ≤1 week (oral anticoagulant + aspirin + clopidogrel) followed by dual therapy (oral anticoagulant + clopidogrel) are recommended.28
Edoxaban and the ENTRUST AF-PCI Trial
The Edoxaban Treatment Versus Vitamin K Antagonist in Patients With AF Undergoing PCI (ENTRUST AF-PCI) trial assessed edoxaban in patients with NVAF requiring PCI (CHA2DS2-VASc score 4.0).29
Overall 1,506 patients (52% after an acute coronary syndrome and 48% after elective PCI) were randomised up to 5 days after PCI in two arms. The experimental regimen was edoxaban 60 mg once daily (or 30 mg once daily if creatinine clearance was ≤50 ml/min, weight was ≤60 kg, or if the patient received P-glycoprotein inhibitors). The control arm was triple therapy with VKA + P2Y12 inhibitor + low-dose aspirin (aspirin was prescribed from 1 to 12 months according to the ischaemic/bleeding risk; Figure 4).
After a 12-month follow-up, the primary outcome (major bleeding or non-major but clinically relevant bleeding) occurred in 128 of 751 patients (annualised event rate 20.7%) with the edoxaban regimen and 152 of 755 patients (annualised event rate 25.6%) patients with the VKA regimen, resulting in a HR of 0.83 (95% CI [0.65–1.05]; p=0.001 for non-inferiority; p=0.1154 for superiority) for edoxaban. At 12 months, there was no difference in the main efficacy endpoint (composite of cardiovascular death, stroke, systemic embolic events, MI, definite stent thrombosis) with an annualised event rate of 7.3% for patients receiving the edoxaban regimen compared with 6.9% for patients receiving the VKA regimen, resulting in a HR of 1.06 (95% CI [0.71–1.69]) for edoxaban.
The ENTRUST AF-PCI trial was the fourth trial showing the safety of a dual therapy combining a DOAC with a P2Y12 inhibitor (mainly clopidogrel) on bleeding outcomes. However, this trial – similar to the three previously described – was underpowered to demonstrate a potential efficacy on ischaemic coronary events.
Meta-analysis of the Four Randomised Clinical Trials
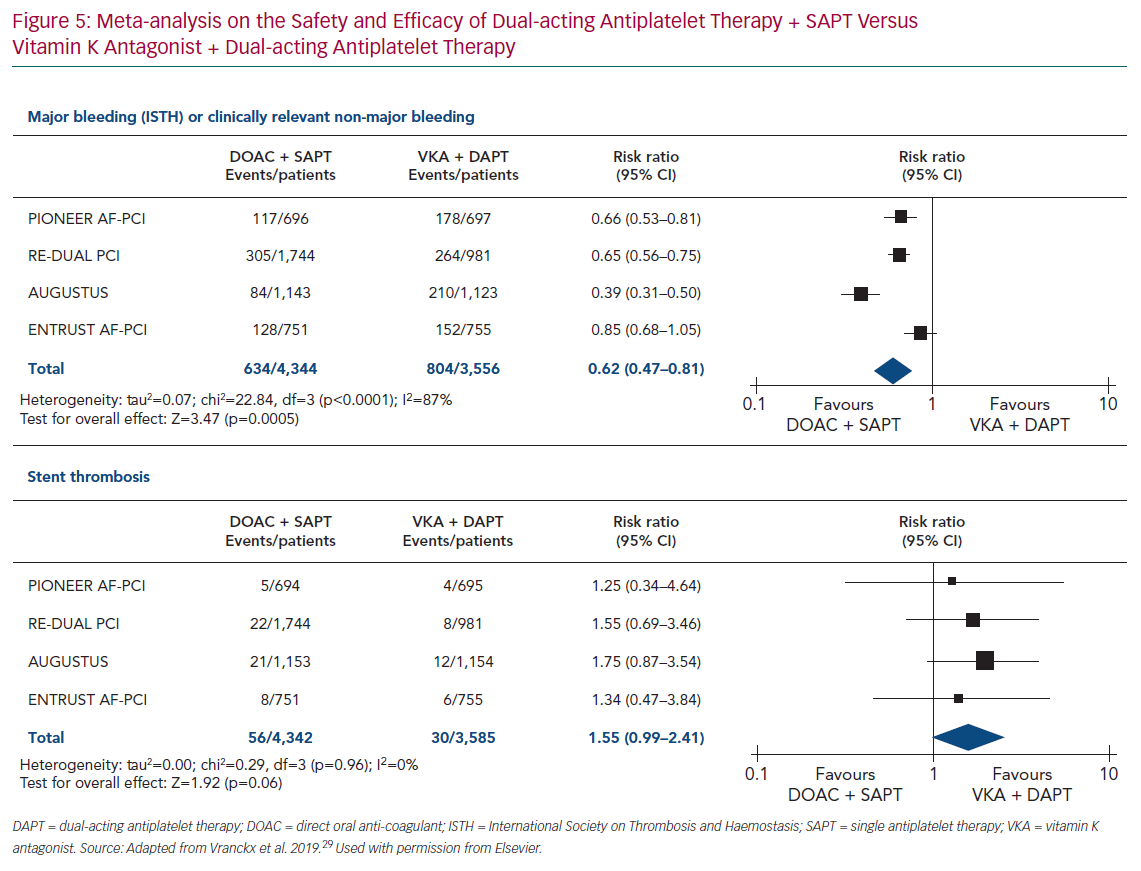
A meta-analysis of the four trials (PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS and ENTRUST AF-PCI) assessed the dual antithrombotic therapy using DOACs with SAPT versus triple antithrombotic therapy using VKA with DAPT.29 The results confirmed the safety of dual antithrombotic therapy with DOAC over triple antithrombotic therapy with VKA on bleeding outcomes with a risk ratio of 0.62 (95% CI [0.47–0.81]; p=0.0005; Figure 5).
This meta-analysis showed a trend towards an increased risk of stent thrombosis with a risk ratio of 1.55 (95% CI [0.99–2.41]; p=0.06) for dual antithrombotic therapy using DOACs versus triple antithrombotic therapy using VKA (Figure 5). These results are in line with European Society of Cardiology (ESC) guidelines, where aspirin in addition to clopidogrel is recommended after PCI for patients with oral anticoagulant (DOAC or VKA), with the duration of aspirin depending on the ischaemic and bleeding risks. One year after PCI, oral anticoagulant without antiplatelet therapy is recommended.28
Direct-acting Oral Anticoagulants and Atherosclerotic Plaque Progression
DOACs could become an additional treatment of the secondary prevention of CCS, not only because of their antithrombotic effect but also because of their potential to limit atheromatous plaque progression. Experimental data suggest that thrombin may have pleiotropic actions including inducing oxidative stress, increasing endothelial dysfunction, vascular inflammation, leucocyte adhesion, rolling, and migration on the activated endothelium and may participate in the early stages of atherosclerotic plaque formation as well as accelerating plaque progression and destabilisation.30,31
Experimental studies evaluating the effect of DOACs on the growth of the atherosclerotic plaque in hypercholesterolaemic ApoE−/− mice demonstrate that dabigatran reduces inflammation, improves cell endothelial function, decreases oxidative stress and reduces atheromatous plaque initiation and progression.32,33 Similar findings have been reported for rivaroxaban.34,35
These experimental findings have yet to be translated clinically. In patients with NVAF, rivaroxaban treatment was associated with a lower total and calcified plaque volume evaluated by coronary CT angiography compared with warfarin, which may explain cardiovascular event reduction associated with rivaroxaban treatment.36 A similar study using apixaban is currently ongoing.37
Direct-acting Oral Anticoagulants as Secondary Prevention in Chronic Coronary Syndromes
The COMPASS Trial
DOACs are easier to use, safer than and as effective as VKAs for thromboprophylaxis in patients with NVAF. Therefore, the question regarding the role of oral anticoagulants in the secondary prevention of atherothrombotic events has been raised.
To date, the only published results in humans are for rivaroxaban. The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial evaluated whether low-dose rivaroxaban alone or in addition to aspirin was safer and more effective than aspirin monotherapy in stable cardiovascular disease.38
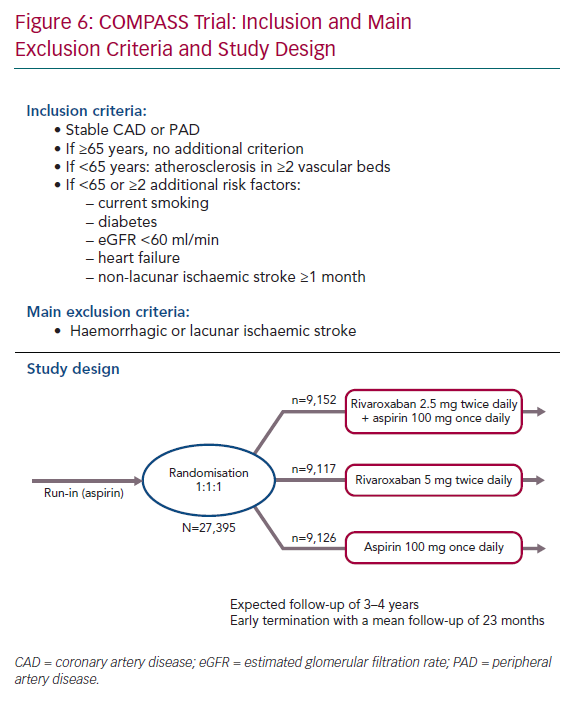
Between 2013 and 2016, this double-blind trial enrolled 27,395 patients with stable cardiovascular disease, including 90% with CCS and 27% with peripheral arterial disease (PAD). To be enrolled, patients had to be at a high cardiovascular risk. If patients were ≥65 years old, no additional inclusion criteria were needed. Patients <65 years old required the presence of atherosclerosis involving at least two vascular beds or two or more additional risk factors: current smoker, diabetes, moderate chronic renal insufficiency, heart failure or non-lacunar ischaemic stroke (≥1 month previously; Figure 6).
Patients were randomised in a 1:1:1 ratio to receive one of the following three regimens:
- Rivaroxaban 2.5 mg twice daily + aspirin 100 mg once daily, long term.
- Rivaroxaban 5 mg twice daily, long term.
- Aspirin 100 mg once daily (control).
The study was prematurely stopped by the data safety monitoring board following interim analysis that showed a significant improvement in the primary composite endpoint, as well as all-cause death.
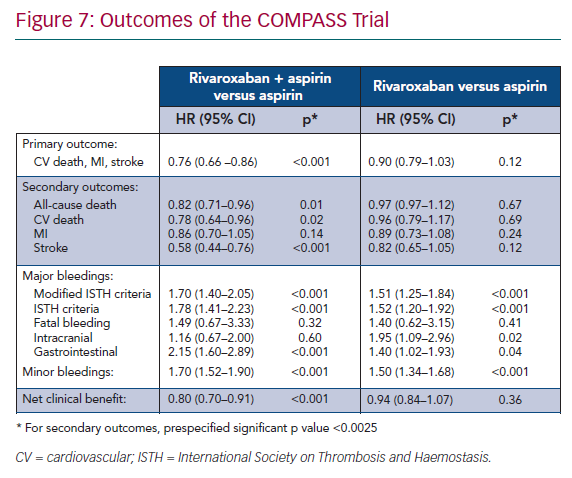
The primary efficacy composite outcome of cardiovascular death, stroke or MI, was significantly lower in the rivaroxaban 2.5 mg twice daily + aspirin group than in the aspirin monotherapy group (4.1% and 5.4%, respectively; HR 0.76; 95% CI [0.66–0.86]; p<0.001). These results were driven by a decrease in cardiovascular death and stroke. The rate of all-cause death was also significantly lower (Figure 7).39
The main safety outcome was major bleeding defined by modified ISTH criteria including all bleeding that led to presentation at an acute care facility or hospitalisation. It was higher in the rivaroxaban 2.5 mg twice daily + aspirin group than in the aspirin monotherapy group (3.1% and 1.9%, respectively; HR 1.70; 95% CI [1.40–2.05]; p<0.001). This outcome was driven by bleeding leading to acute care facility presentation or blood transfusion. Importantly, there was no significant difference in the rate of fatal bleeding, intracranial bleeding, or symptomatic bleeding into a critical organ (Figure 7).39
The subjective net clinical benefit outcome taking into account cardiovascular death, stroke, MI, fatal bleeding or symptomatic bleeding into a critical organ was in favour of rivaroxaban + aspirin therapy (HR 0.80; 95% CI [0.70–0.91]; p<0.00.1; Figure 7) compared to aspirin alone.39 However, the benefit/risk ratio was unfavourable for rivaroxaban 5 mg twice-daily monotherapy compared with aspirin monotherapy. There was no significant improvement in the efficacy outcome but there was a significant increase in major bleeding, advocating against this strategy (Figure 7).39
The substudy of patients with CCS in the COMPASS trial, comprising 90.6% of the entire population and performed using the same design, showed similar results.40
Therefore, despite early termination – possibly overestimating the treatment effect – the findings of the COMPASS study suggested that rivaroxaban 2.5 mg twice daily may become an additional treatment for CCS in selected patients at high ischaemic risk.
The COMMANDER HF Trial
Decompensated heart failure is associated with an activation of thrombin-related pathways. Thus, patients with CCS with worsening heart failure may be a subpopulation that benefits from the addition of a DOAC. The Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure (COMMANDER HF) assessed the association of low dose of rivaroxaban and aspirin in patients with CCS and chronic heart failure (left ventricular ejection fraction ≤40%) and recent decompensation.41
The main exclusion criteria were AF, recent acute MI or revascularisation (<21 days), stroke (<3 months), bleeding risk, anaemia (haemoglobin <8 g/dl) or thrombocytopaenia (platelets <50,000/µl). A total of 5,022 patients were enrolled from 2013 to 2017 in this double-blind multicentre trial, and randomly assigned to receive rivaroxaban 2.5 mg twice daily or placebo in addition to standard care therapy.There was no significant difference in the occurrence of the primary efficacy outcome (composite of MI, stroke and all-cause death) and the secondary safety outcome (fatal or major bleeding) over a median follow-up of 21.1 months.
Direct-acting Oral Anticoagulants in International Guidelines
The 2019 ESC Guidelines for the diagnosis and management of CCS provide the following recommendations:28
- When oral anticoagulation is initiated in a patient with NVAF, a DOAC is recommended in preference to a VKA (recommendation IA).
- Aspirin 75–100 mg daily (or clopidogrel 75 mg daily) may be considered in addition to long-term oral anticoagulant therapy in patients with NVAF and CCS at high risk of recurrent MI (prior MI, multivessel disease, diabetes, chronic kidney disease with estimated glomerular filtration rate <60 ml/min/1.73m2, PAD) NVAF, but not high risk of bleeding (recommendation IIaB).
- For PCI in patients with DOAC, it is recommended that peri-procedural aspirin and clopidogrel are administered to patients undergoing PCI (recommendation IC).
- After PCI, in patients eligible for a DOAC, it is recommended that a DOAC (apixaban 5 mg twice daily, dabigatran 150 mg twice daily, edoxaban 60 mg once daily, or rivaroxaban 20 mg once daily) is used in preference to a VKA in combination with antiplatelet therapy (recommendation IA).
- After PCI, when rivaroxaban or dabigatran are used and concerns about high bleeding risk prevail over concerns about stent thrombosis or ischaemic stroke, lower DOAC doses should be considered for the duration of concomitant SAPT or DAPT as follows: rivaroxaban 15 mg once daily, dabigatran 110 mg twice daily (recommendation IIaB).
- After PCI, early cessation (≤1 week) of aspirin and continuation of dual therapy with an oral anticoagulant and clopidogrel should be considered if the risk of stent thrombosis is low, or if concerns about bleeding risk prevail over concerns about the risk of stent thrombosis irrespective of the type of stent used (recommendation IIaB).
- After PCI, triple therapy with aspirin, clopidogrel, and an oral anticoagulant for ≥1 month should be considered when the risk of stent thrombosis outweighs the bleeding risk, with the total duration (≤6 months) decided according to assessment of these risks (recommendation IIaC).
- The use of ticagrelor or prasugrel is not recommended as part of triple antithrombotic therapy with aspirin and an oral anticoagulant (recommendation III).
Conclusion
In patients with NVAF treated with DOACs, switching to a VKA regimen is not required when a PCI is needed. Short-term triple antithrombotic therapy (DOAC + aspirin + clopidogrel) up to 3 months followed by dual antithrombotic therapy (DOAC + clopidogrel) up to 1 year seems to be the best strategy, compared with triple antithrombotic therapy (VKA + aspirin + clopidogrel).
DOACS may become an accepted secondary prevention therapy as suggested by the COMPASS study. However, before DOACs become a cornerstone of the management of selected patients with CCS, further studies are needed to address some issues. Identifying which patients will derive the highest ischaemic benefit with the lowest haemorrhagic risk needs to be determined. The additional benefit on the recurrence of major cardiovascular events and death with rivaroxaban in such patients is offset by the higher rate of bleeding events. In that case patients should not move to this strategy, particularly if they are stable, asymptomatic and risk the occurrence of both major and minor bleeding. The potential use of DOACs in combination with new antiplatelet drugs is not known. Moreover adherence may be challenging because of the need for a twice-daily dosing regimen. All these factors will have to be discussed individually with each patient for optimal individualised care that improves both outcomes and quality of life (i.e. taking into consideration the ischaemia/bleeding ratio).
Another issue will be the cost of this additional new therapy. Given the high number patients potentially eligible for low dose rivaroxaban in addition to aspirin,42 the clinical/cost ratio compared with the low cost of the aspirin alone will have to be evaluated. This is particularly relevant given that the absolute risk reduction is quite low (the number needed to treat to avoid one primary outcome is 77).