Patients with AF who require anticoagulation are typically aged 65;years or above or have at least one comorbidity.1,2 Therefore, clinicians inevitably encounter the issue of anticoagulation use and selection in elderly patients and those with comorbidities during clinical practice. An analysis of more than 70,000 patients from four pivotal studies investigating non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in AF showed that nearly half of all deaths were attributable to cardiovascular causes. In contrast, the combined number of deaths resulting from stroke/systemic embolism and haemorrhage accounted for only 11.3% of the total mortality.3 By implementing the Atrial Fibrillation Better Care (ABC) model recommended by the European Society of Cardiology (ESC), which encompasses comorbidity management, symptom control and stroke prevention, the estimated risk reductions for stroke, bleeding and all-cause mortality were 31%, 45% and 58%, respectively.2,4 These findings emphasise the need to address patient-specific factors when managing AF, including the selection of anticoagulation options, beyond stroke prevention.
The majority of AF patients are elderly and commonly have comorbidities such as diabetes, renal impairment and coronary artery disease. This population is also at high risk of both thrombosis and bleeding when anticoagulation is administered. Consequently, the use of anticoagulation in these patients presents numerous clinical challenges. Furthermore, there is an unmet need in the management of patients with prosthetic valves, particularly in the context of biological valves where the selection of anticoagulation remains uncertain. In Part 2 of this review, we provide an in-depth discussion of the available anticoagulation options specifically tailored to these patient populations and address their unique considerations and challenges.
Renal Impairment
In patients with AF, the prevalence of chronic kidney disease (CKD) was found to be 10.8%.5 The prevalence of CKD is known to increase with age. In a study of elderly patients with AF in Vietnam, the rate of abnormal renal function was 22.2%.6 In two separate studies on Vietnamese patients, the prevalence of CKD after the age of 40;years was 3.1%, and 9.2% of CKD patients were found to have AF.7,8 AF patients with CKD have a higher risk of stroke/systemic embolism (SE; HR 1.49; 95% CI [1.38–1.59]; p<0.001) and anticoagulant-related bleeding compared with those without CKD (HR 1.33; 95% CI [1.16–1.53]; p<0.001).9 The risk of stroke and death in patients with AF increases with the severity of CKD.10 Cardiovascular diseases, including ischaemic heart disease and stroke, are the leading cause of death in AF patients with CKD.11 Among Vietnamese patients on dialysis, stroke, heart failure and MI accounted for 24.2%, 15.7% and 6.2% of deaths, respectively.12 Concerns were raised in the RE-LY study and in meta-analyses of randomised controlled trials (RCTs) about the increased risk of MI associated with dabigatran.13,14 Therefore, when choosing an anticoagulant for this population, considerations should go beyond stroke prevention and mitigation of the bleeding risk to include preservation of renal function and reduction of major adverse cardiac events. Figure 1 presents the criteria for selecting NOACs in renal impairment.
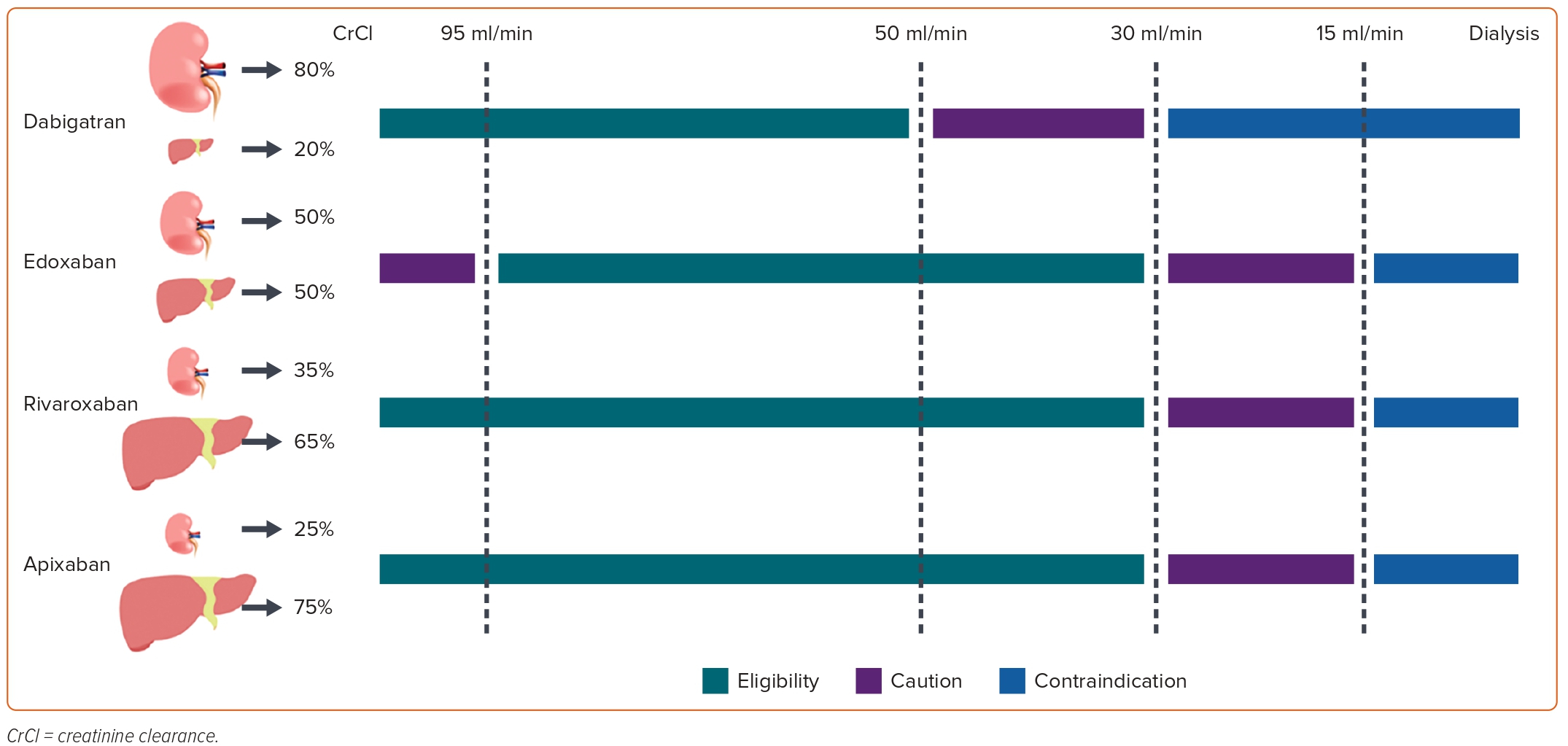
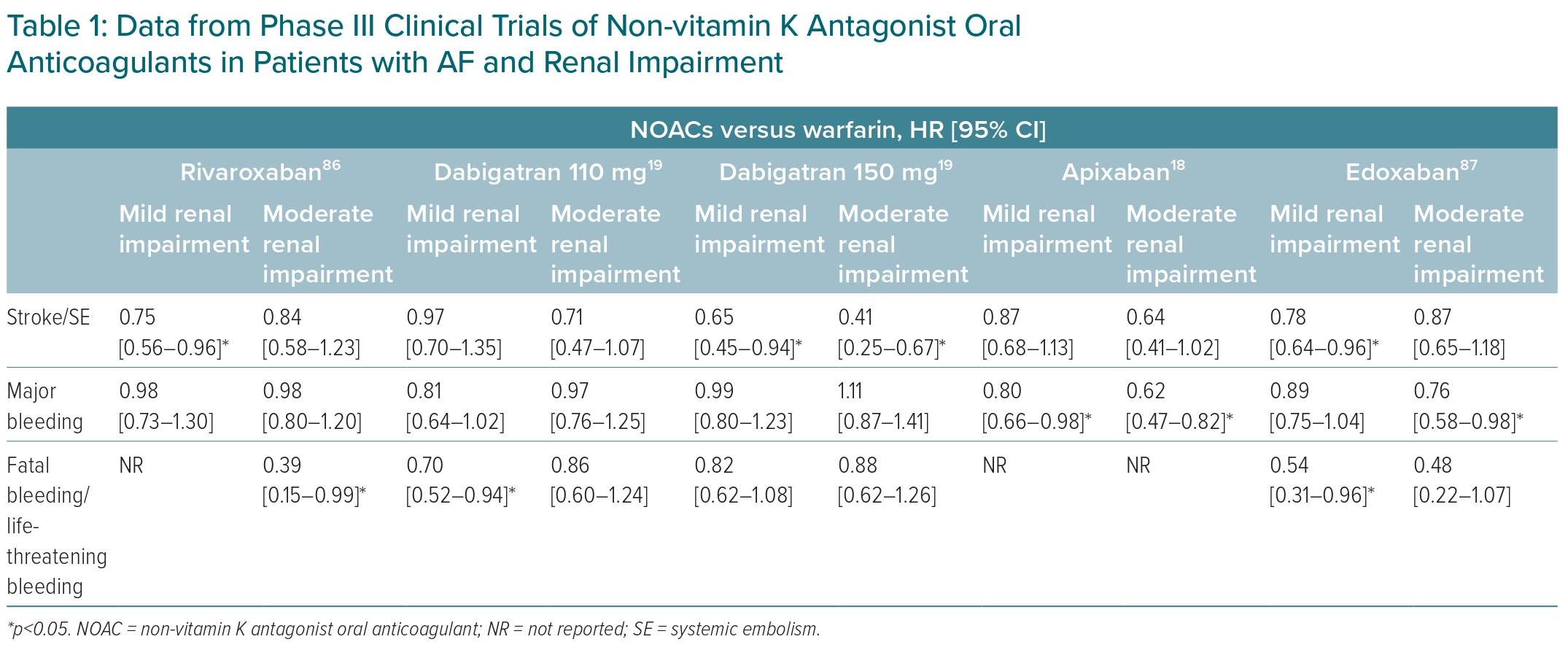
NOACs have varying renal clearance rates, and the use of these medications in renal impairment varies as well. It is important to note that accelerated CKD progression and acute kidney injury can occur in patients undergoing anticoagulation therapy.15 Thus, it is recommended to use NOACs with low rates of renal clearance and a wide treatment range. The rate of renal clearance and suitability for NOACs are summarised in Figure 1. A meta-analysis of RCTs showed that NOACs were more effective than warfarin in preventing stroke/SE events in patients with mild renal impairment and creatinine clearance (CrCl) 50–80 ml/min, with an OR of 0.78 (95% CI [0.67–0.91]; p<0.05), and moderate renal impairment (CrCl 30–50 ml/min) with an OR of 0.80 (95% CI [0.67–0.95]; p<0.05). The risk of major bleeding also appeared to be lower in patients with mild-to-moderate renal impairment taking NOACs.16 Data from Phase III trials of NOACs in patients with renal impairment are given in Table 1.
An important consideration in the management of AF patients with CKD is the slowing of the progression of CKD, given that a worsening renal function is linked to a higher risk of overall mortality in AF patients.17 Accelerated CKD progression and acute kidney injury can occur in patients on anticoagulants.15 In the post hoc analysis of ROCKET-AF, rivaroxaban significantly reduced risk of stroke/SE (HR 0.50; 95% CI [0.27–0.93]), composite stroke/embolism/vascular death/MI events (HR 0.67; 95% CI [0.46–0.97]), and had a lower trend of MI (HR 0.45; 95% CI [0.18–1.10]) compared with warfarin in patients with worsening renal function.17 In the subgroup analysis of the ARISTOTLE trial, there was no significant difference between apixaban and warfarin in terms of the stroke/SE risk in patients with worsening renal function (HR 0.83; 95% CI [0.52–1.32]).18 Table 2 lists NOAC data from phase III trials on patients with worsening renal function.17–19
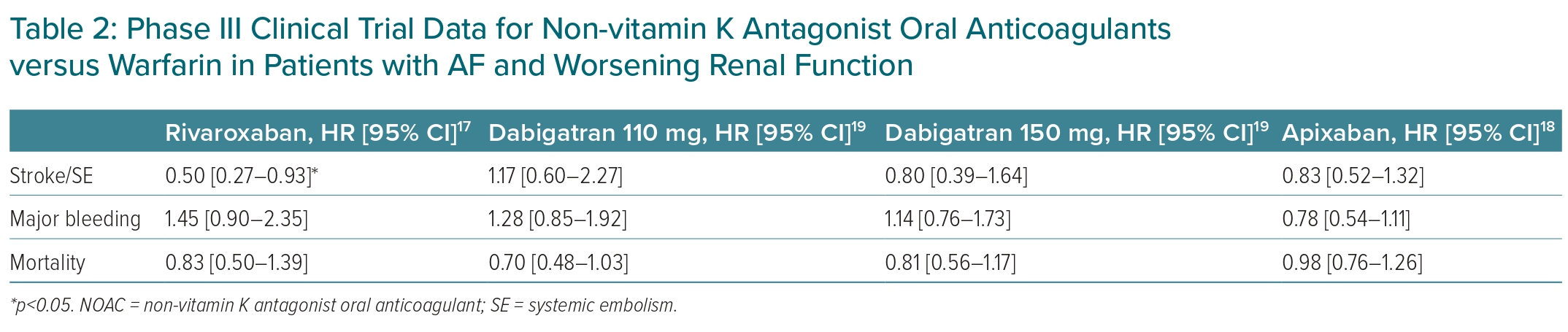
The benefit of NOACs on renal outcomes has also been demonstrated in several observational studies. A study by Yao et al. compared rivaroxaban, apixaban and dabigatran with warfarin across four renal outcomes, namely ≥30% decrease in the estimated glomerular filtration rate, a doubling of serum creatinine levels, acute kidney injury, and kidney failure.20 Of the four renal outcomes, rivaroxaban reduced the risk of three, while dabigatran reduced the risk of two when compared with warfarin. Apixaban did not significantly reduce renal outcomes compared with warfarin.20 In another retrospective analysis involving 12,000 patients, rivaroxaban, when compared with dabigatran, significantly reduced the risk of a decrease in estimated glomerular filtration rate of 30 ml/min/1.73 m2 (HR 0.29; 95% CI [0.13–0.66]; p=0.003) and the risk of a doubling of serum creatinine (HR 0.62; 95% CI [0.40–0.95]; p=0.030). Rivaroxaban showed a greater tendency to reduce the risk of renal outcomes compared with apixaban but the difference was not statistically significant.21 Data on the effect of edoxaban on renal outcomes are limited.
In summary, we recommend rivaroxaban and apixaban as the preferred treatment options and edoxaban as an alternative for stroke prevention in patients with AF and renal impairment.
Patients with Diabetes
With a prevalence of 6.0%, diabetes is one of the most common non-communicable diseases in Vietnam.22 According to multinational data, the prevalence of diabetes in patients with AF is 22.2%, and this proportion ranges from 15.7% to 44.4% in Vietnam.5–7 Diabetes is a well-established risk factor for AF through multiple pathomechanisms.23 Diabetes increases the risk of all-cause mortality and cardiovascular mortality, as well as reducing the quality of life in patients with AF. However, it is surprising that there is no increased risk of thromboembolic events or haemorrhagic hospitalisation compared with those without diabetes.24 A retrospective analysis of 116,049 patients with AF and diabetes, between the years 2010 and 2019, found that the total number of vascular deaths (10,239 events) was greater than the number of stroke/SE events (4,494 events) and major bleeding events (3,521 events).25 Therefore, when selecting anti-thrombotic agents for patients with AF and diabetes, it is important to not only consider the risk of stroke and major bleeding but also to mitigate the risk of vascular death. Renal complications and amputations are other major burdens for patients with diabetes and are associated with an increased risk of mortality globally.26,27 Additionally, in Vietnam, cardiovascular events are a major burden, accounting for about half of the cost of diabetes care.28
The benefit of NOACs over warfarin in patients with AF with diabetes has been demonstrated in a subgroup of pivotal studies.29–32 A meta-analysis of these subgroups showed that NOACs were superior to warfarin in preventing stroke/SE (HR 0.80; 95% CI [0.69–0.93]) without an increased risk of major bleeding (HR 0.95; 95% CI [0.75–1.20]).33 In the indirect comparison, there was no significant difference between NOACs in the risk of stroke and major bleeding in patients with AF and diabetes.34 Rivaroxaban was the only agent that reduced the risk of vascular death in diabetic patients with AF compared with warfarin (HR 0.80; 95% CI [0.64–0.99]).30 Using a risk index based on data from Phase III studies, an analysis of the net clinical benefit, including stroke, SE, vascular death and major bleeding, favoured rivaroxaban over other NOACs.35
When it comes to individual NOAC data, there are a few aspects to consider. Except for apixaban, the bleeding risk for NOACs compared with warfarin is consistent regardless of baseline diabetes status. The advantage of apixaban in reducing the risk of major bleeding over warfarin has not been observed in patients with diabetes.36 This has also been emphasised in international clinical guidelines for AF management.2 MI is the leading cause of death in patients with diabetes. Data from Phase III studies show that NOACs (when compared with warfarin) reduce the risk of MI, but this was not statistically significant (HR 0.82; 95% CI [0.66–1.02]; p=0.07).33 Although there are no published data on the risk of MI in patients with diabetes in the RE-LY study, a meta-analysis of RCTs on AF raises concerns about a possible association between dabigatran and an increased risk of MI compared with warfarin (HR 1.38; 95% CI [1.14–1.67]).37
The benefits of using NOACs in diabetic nephropathy and limb events are becoming increasingly evident in recent real-world studies. CALLIPER, a retrospective, observational study, evaluated the effects of rivaroxaban 15 mg and warfarin on renal function. In that analysis, rivaroxaban showed a 47% reduction in the risk of worsening renal function in patients with diabetes compared with warfarin.38 A large retrospective cohort study of 21,682 patients with AF and diabetes showed that treatment with rivaroxaban reduced the risk of acute kidney injury by 17% and the risk of progression to end-stage CKD or haemodialysis by 18% compared with warfarin treatment.39 Another retrospective analysis of more than 24,000 patients showed that treatment with rivaroxaban reduced the risk of major adverse limb events by 63% and the risk of major limb amputation by 80%.40 Furthermore, large RCTs demonstrated a similar result on the efficacy of rivaroxaban in reducing the risk of major adverse limb events.41
In summary, we recommend rivaroxaban as the preferred choice and edoxaban as an alternative for stroke prevention in patients with AF and diabetes.
Patients with Coronary Artery Disease
Coronary artery disease (CAD) and AF are prevalent cardiovascular diseases that share common risk factors. A study of 236 patients with AF in Vietnam reported that the prevalence of CAD was 30.1%.42 Additionally, the prevalence of AF in patients with acute MI has been reported to be 6.5%.43 Antiplatelet agents and anticoagulants are the cornerstones of treatment for CAD and AF, respectively. However, the situation is much more complicated when these two conditions coexist. In this situation, clinicians must carefully weigh the risks of stroke/SE, coronary ischaemic events and major bleeding.
In a clinical setting, one may encounter a patient with AF who requires either an urgent percutaneous coronary intervention (PCI) or an elective intervention. According to international clinical guidelines, the recommended short-term therapy for this patient population is triple anti-thrombotic therapy (TAT), which consists of dual antiplatelet therapy and anticoagulation. After this initial period, the patient should receive a dual anti-thrombotic combination of anticoagulants and P2Y12 inhibitors for 6–12 months. After 6–12 months, anticoagulant monotherapy can be considered a viable option (Figure 2).2,44
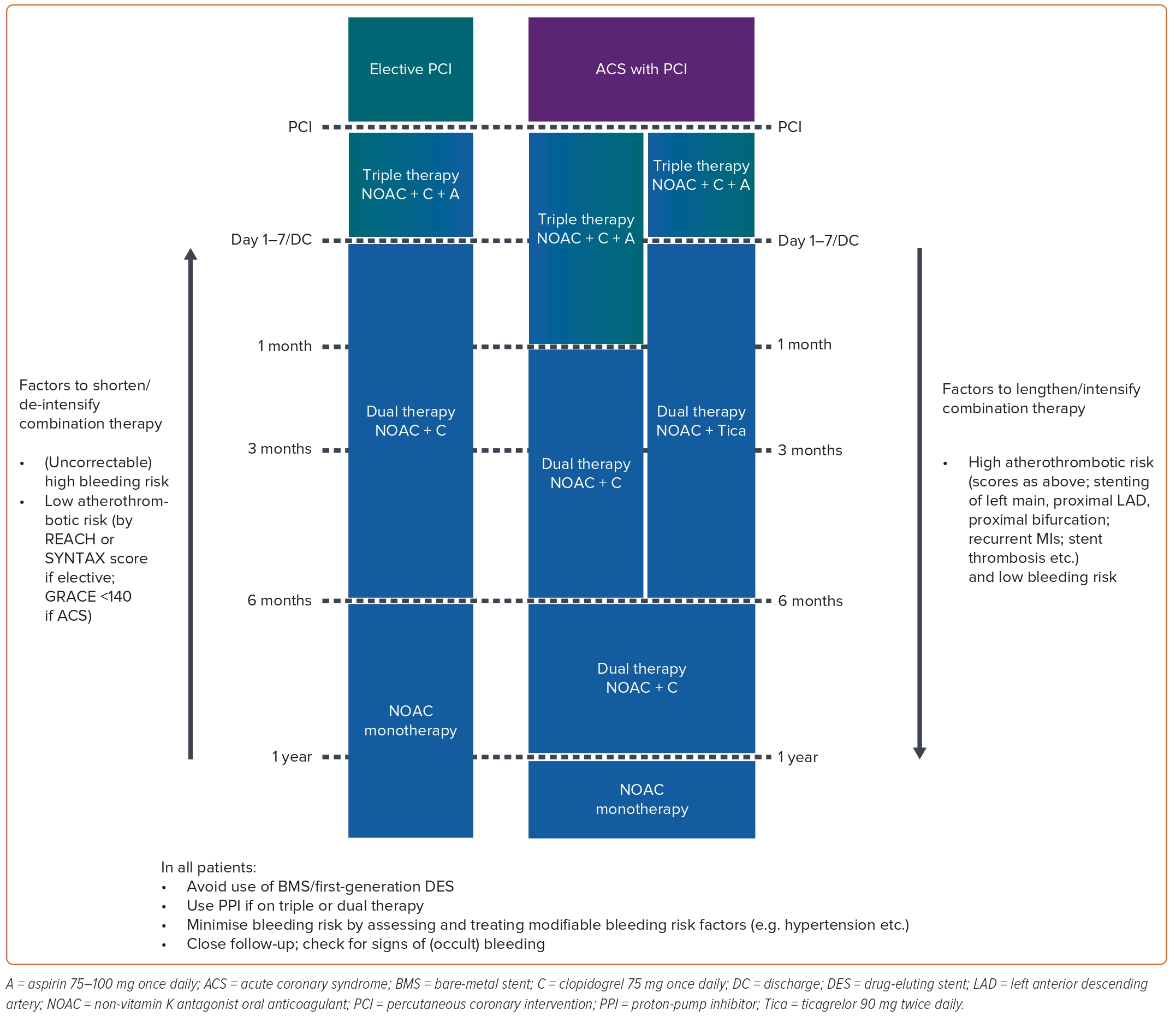
These recommendations are based on the results of four pivotal trials on NOACs in patients with AF undergoing PCI: Pioneer-AF PCI (rivaroxaban), REDUAL-PCI (dabigatran), ENTRUST AF-PCI (edoxaban) and AUGUSTUS (apixaban).45–48 The primary endpoint of these four studies was the International Society on Thrombosis and Haemostasis major bleeding or clinically relevant non-major bleeding.45–48 In a meta-analysis of these studies, a NOAC-based dual anti-thrombotic therapy (DAT) regimen reduced the risk of the primary endpoint by 38% (14.6% versus 22.6%; RR 0.62; 95% CI [0.47–0.81]; p<0.0001) and the risk of major bleeding by 41% (4.3% versus 6.9%; RR 0.59; 95% CI [0.41–0.83]; p<0.0001) compared with vitamin K antagonist (VKA)-based TAT.49 However, edoxaban was the only NOAC to fail superiority tests in both the primary endpoint (HR 0.83; 95% CI [0.65–1.05]; p=0.1154 for superiority) and major bleeding (HR 0.95; 95% CI [0.63–1.42]) compared with the VKA-based TAT.48 Besides the risk of bleeding, the risks of MI, stent thrombosis and stroke should also be considered when using a NOAC-based DAT regimen. Unfortunately, all four studies were underpowered to compare regimen efficacy.45–48 The meta-analysis of these studies raised concerns about the risk of MI (RR 1.18; 95% CI [0.93–1.52]) and stent thrombosis (RR 1.55; 95% CI [0.99–2.41]) with the use of NOAC-based DAT regimens, despite comparable stroke prevention efficacy (RR 0.89; 95% CI [0.58–1.36]) versus VKA-based TAT.49 Therefore, the selection of the anti-thrombotic regimen and the duration of therapy must be judiciously considered, considering the individual’s risk of stroke, coronary ischaemia and bleeding events.
For patients with AF who have had successful PCI after more than 12 months or who have AF and chronic coronary syndromes (CCS), NOACs can be used as monotherapy.2,44 However, these recommendations are not based on strong evidence from RCTs. A meta-analysis of subgroups of patients with CAD in pivotal trials for stroke prevention in patients with AF was conducted.50 The results showed that NOACs were non-inferior to warfarin for the following outcomes: stroke/SE (HR 0.76; 95% CI [0.56–1.04]), major bleeding (HR 0.92; 95% CI [0.65–1.32]) and MI (HR 0.95; 95% CI [0.62–1.44]).50 It should be noted, however, that in the group of AF patients with CAD in Phase III trials, approximately more than 40% of patients were taking antiplatelet agents. Therefore, the important clinical question is whether patients with AF and CCS should be treated with NOAC monotherapy or in combination with antiplatelet agents.
An RCT compared anticoagulant therapy as monotherapy and in combination with antiplatelet agents in patients with AF who had undergone PCI more than 1 year previously. However, that study was terminated prematurely and did not have conclusive outcomes.51 AFIRE, an open-label RCT involving 2,240 patients with AF with stable CAD, showed that rivaroxaban monotherapy was non-inferior to rivaroxaban plus an antiplatelet agent. The primary efficacy endpoints included a composite of stroke/SE, MI, unstable angina requiring revascularisation, or death from any cause (HR 0.72; 95% CI [0.55–0.95]; p<0.001 for non-inferiority).52 Rivaroxaban monotherapy also reduced the risk of major bleeding by 41% compared with combination therapy (HR 0.59; 95% CI [0.39–0.89]; p=0.01).52 In a post hoc analysis, it was established that rivaroxaban significantly reduced the risk of cardiovascular events and bleeding events compared with combination therapy (HR 0.62; 95% CI [0.48–0.80]; p<0.001).53
In summary, we recommend a reduced dose of rivaroxaban (15 mg for patients with CrCl 50 ml/min and 10 mg for patients with CrCl 15–49 ml/min), dabigatran (110 mg twice daily), and apixaban for patients with AF who are undergoing PCI in the next 6–12 months. In patients with AF with CCS, rivaroxaban is the preferred choice.
Elderly Patients
Vietnam is going through a demographic shift towards an ageing population. It is projected that by 2050 the proportion of people older than 60 years of age in Vietnam will be threefold that of the current elderly population.54 Advanced age is a well-established risk factor for AF, and the incidence of AF increases with age.55 The prevalence of AF in Vietnamese people <55 years of age is 0.1%, 3.9% in those older than 60 years of age, and 9% in those older than 80 years of age.6,7 Elderly patients have a higher risk of stroke and bleeding with anticoagulants than younger patients.56–59 In the CHA2DS2-VASc score, an age cut-off of 65 years is commonly used to determine the initiation of anticoagulation in elderly patients. However, for the Asian population, this cut-off threshold may require reconsideration. Studies conducted in the Chinese and Taiwanese populations, including a study with nearly 190,000 Taiwanese patients, found a significant increase in stroke risk after the age of 50 years, with the stroke risk being more than threefold higher in individuals aged 50 years and above.60,61 Another study of more than 400,000 Korean patients by Kim et al. found that AF patients aged 55–59 years without risk factors had a similar stroke risk to those with one risk factor on the CHA2DS2-VASc score.62 These findings suggest that in the Asian population, there may be a need for earlier initiation of anticoagulation, indicating the importance of considering age thresholds specific to this population. However, further studies are required to establish a more precise and appropriate age threshold for initiating anticoagulation in Asian patients. The clinical outcomes in elderly patients who have had a stroke or a haemorrhagic event are unsatisfactory. Stroke and haemorrhagic mortality rates were threefold higher in patients ≥75 years old who received high-dose edoxaban than in patients <65 years old in ENGAGE-TIMI 48.57 Thus, in addition to the risk of stroke and major bleeding, the severity of events in patients on anticoagulants should also be considered.
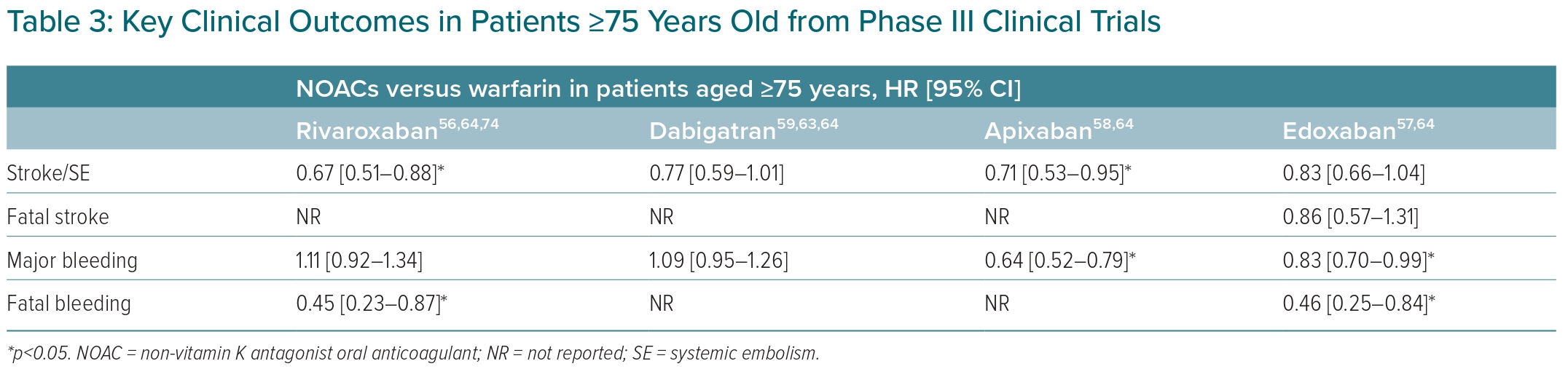
In Phase III trials, all four NOACs had more favourable outcomes compared with warfarin in the elderly population. For stroke/SE prevention, rivaroxaban (HR 0.67; 95% CI [0.51–0.88]) and apixaban (HR 0.71; 95% CI [0.53–0.95]) were superior to warfarin. Apixaban (HR 0.64; 95% CI [0.52–0.79]) and edoxaban (HR 0.83; 95% CI [0.70–0.99]) were associated with a reduced risk of major bleeding compared with warfarin. Rivaroxaban (HR 0.45; 95% CI [0.23–0.87]) and edoxaban (HR 0.46; 95% CI [0.25–0.84]) were associated with a reduced risk of fatal bleeding compared with warfarin. A few key clinical outcomes in subgroups of patients ≥75 years old are summarised in Table 3.
A major clinical challenge when managing elderly patients is the underuse of anticoagulation therapy. Data from the GARFIED-AF registry showed that 45.2% of AF patients ≥75 years of age were not receiving anticoagulants.65 This proportion of elderly patients in Vietnam is even higher, at 77.8%.6 However, it is important to remember that elderly patients with AF who use anticoagulants will derive a greater net clinical benefit than those who do not.66 The most common reason for the low usability of anticoagulants is fear of bleeding, making it the primary reason for off-label use of low-dose NOACs in the elderly. The off-label use of low-dose anticoagulation presents more harm than benefit. In an analysis of nearly 15,000 patients, the underdose rates for apixaban, rivaroxaban and dabigatran in elderly patients were 48%, 35.4% and 25.7%, respectively.67 In an analysis of 45,947 patients, those receiving apixaban 2.5 mg twice daily (who did not meet the criteria for dose reduction) had an associated increased risk of ischaemic stroke, major bleeding and mortality, but this dose did not significantly reduce the risk of intracranial haemorrhage.68 As a result, providing anticoagulants to eligible patients and using the appropriate dose is critical to improving clinical outcomes in the elderly.
Elderly patients may have comorbidities, cognitive impairment and complex therapies with multiple prescriptions, which can affect treatment adherence.69 It has been noted that non-adherence to anticoagulants worsens the prognosis for clinical outcomes.70 Therefore, appropriate measures to improve patient adherence should be implemented, such as tailoring treatment to the patient’s clinical profile, patient education, and regular patient communication. A cohort study of more than 200,000 patients with AF showed that adherence and persistence for once-daily anticoagulants were higher than those for twice-daily anticoagulants.71
Another factor to consider in elderly patients is frailty. In a meta-analysis, the prevalence of frailty in the AF population was 39%. Frailty is associated with an increased risk of adverse outcomes and a reduced rate of anticoagulation in patients with AF.72 In a retrospective analysis of frail patients with AF, rivaroxaban reduced the risk of stroke/SE more than warfarin at 2 years (HR 0.68; 95% CI [0.49–0.95]). However, these results were not observed in the group that used apixaban (HR 0.78; 95% CI [0.46–1.35]) or dabigatran (HR 0.94; 95% CI [0.60–1.45]). When compared with warfarin, none of the three NOACs increased the risk of major bleeding.73
In summary, we recommend apixaban, edoxaban and rivaroxaban as the preferred choices for elderly patients with AF.
Patients with Bioprosthetic Mitral Valve
Currently, the Summary of Product Characteristics approved by most health authorities allows the use of NOACs only in non-valvular AF (NVAF).44 However, the term ‘NVAF’ can be misleading, as it implies that a patient with AF does not have valvular heart disease. In pivotal trials of NOACs for stroke prevention in patients with AF, patients with mechanical prosthetic valves or moderate-to-severe mitral stenosis were excluded.13,36,74,75 The results of the INVICTUS study showed that warfarin was effective in reducing thromboembolic events and did not increase major bleeding events compared with rivaroxaban in rheumatic heart disease in patients with AF.76 Thus, it is important to remember that ‘non-valvular’ means without mechanical prosthetic valves or moderate-to-severe mitral stenosis; hence, to avoid misunderstanding, this term should not be used.2
In contrast to patients with mechanical valves, there is some evidence that NOACs should be considered in patients with bioprosthetic valves. The ARISTOTLE subgroup analysis of patients with a history of bioprosthetic valves or valve repairs showed that the risk of stroke/SE (HR 1.71; 95% CI [0.31–9.37]; p=0.53) and major bleeding (HR 0.882; 95% CI [0.31–2.52]; p=0.82) was not significantly different between apixaban and warfarin.77 Similar results were observed in the subgroup analysis of the ENGAGE-TIMI 48 study in patients with bioprosthetic valves.78 The limitation of these two subgroup analyses is that the sample sizes were too small and underpowered to enable assessment of the efficacy and safety criteria. The first published RCT specific for patients with bioprosthetic valve replacement comparing dabigatran and warfarin was terminated prematurely due to insufficient enrolment, and the results were inconclusive.79 The ENAVLE study was an open-label RCT that compared edoxaban and warfarin in 218 patients with AF and with bioprosthetic valves or valve repair.80 Although edoxaban was shown to be non-inferior to warfarin for the primary efficacy endpoint and major bleeding, the study had a few limitations. The relatively short comparison period of only 3 months, and a lower than expected number of events, may have reduced the power of the study.80 RIVER is the most well-designed RCT published comparing NOACs and warfarin in patients with bioprosthetic valves.81 The study involved 1,000 patients with AF with bioprosthetic valves, and outcomes were assessed at 12 months. The study found that rivaroxaban was non-inferior to warfarin for the primary endpoint. The results for stroke (HR 0.25; 95% CI [0.07–0.88]) and cardiovascular/thromboembolic mortality (HR 0.65; 95% CI [0.35–1.20]) also favoured rivaroxaban over warfarin. The risk of any bleeding (HR 0.83; 95% CI [0.59–1.15]) and major bleeding (HR 0.54; 95% CI [0.21–1.35]) was not statistically different between rivaroxaban and warfarin.81
In summary, we recommend rivaroxaban as the preferred choice and edoxaban as an alternative for patients with AF with bioprosthetic valves.
Discussion and Expert Opinion
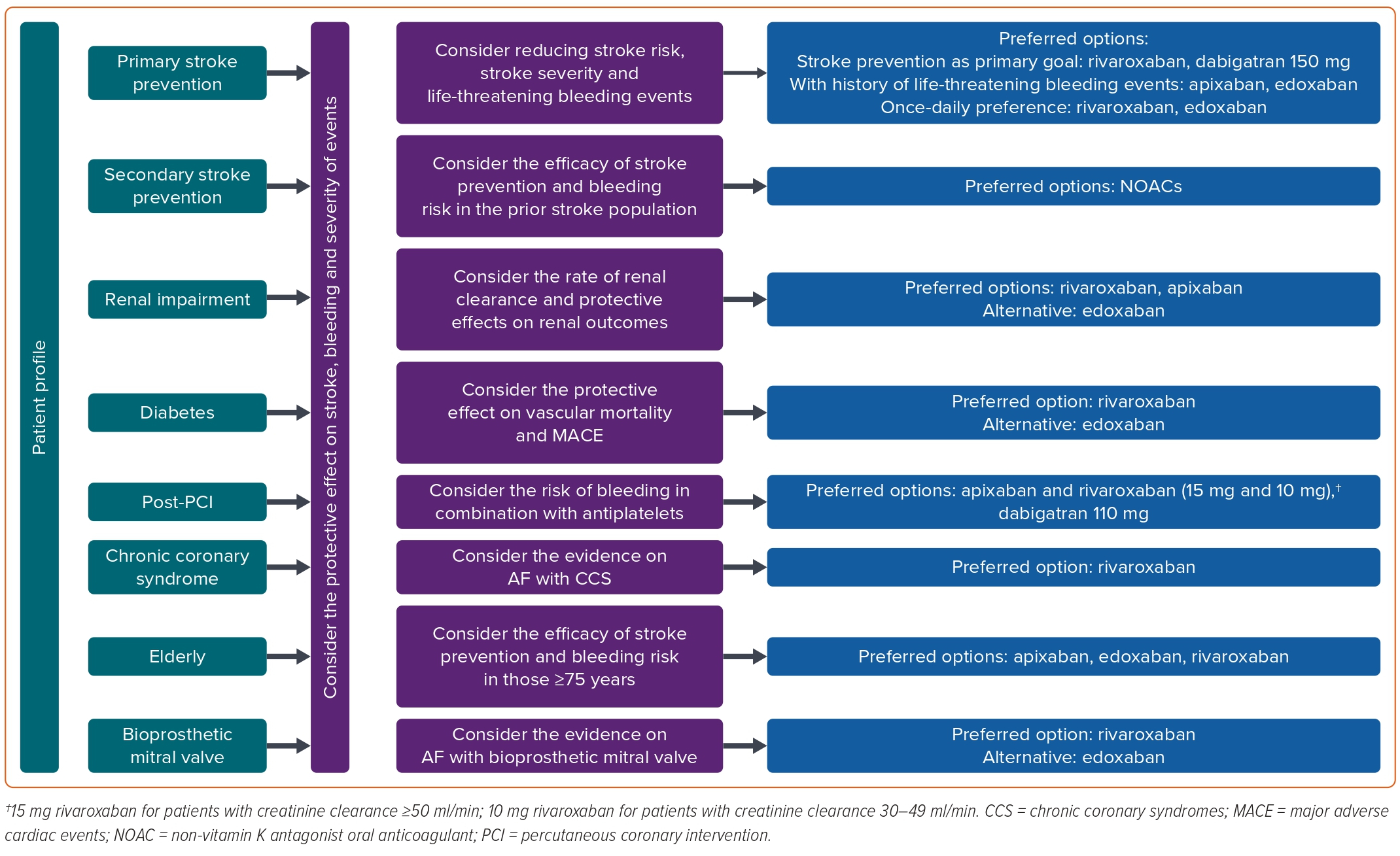
Given the absence of head-to-head RCTs comparing NOACs, it is challenging to make specific recommendations for the selection of a particular NOAC. However, this is an important concern for clinicians in their daily practice. Hence, evidence-based recommendations for the selection of anticoagulants are imperative for the effective clinical management of AF. Adopting a patient-centred approach, clinicians should assess the problems presented by patients in specific clinical contexts. Instead of solely relying on the number of strokes and bleeding events, clinicians should carefully consider all factors and choose the most appropriate anticoagulant to resolve patients’ issues. Our recommendations are primarily based on the subgroup analysis, combined with evidence from real-life practice, and may change as more high-quality evidence becomes available. A summary of recommendations for anticoagulant selection in patients with AF in Part 1 and Part 2 of the review is shown in Figure 3.
Treatment adherence to oral anticoagulants is an ongoing issue in the management of thrombotic diseases.82 In Vietnam, this problem is particularly pronounced, given that only approximately one-third of patients have good adherence to anticoagulation therapy.83 It is crucial to emphasise that patients can benefit from stroke prevention only as long as they maintain regular anticoagulant use. Thus, regardless of the specific anticoagulant selected for a patient, adherence plays a pivotal role in achieving favourable treatment outcomes. Clinical practitioners must actively involve patients in treatment decision-making processes. Apart from considering factors such as medication efficacy and safety, patient preferences should also be taken into account. This collaborative approach ensures that patients and clinicians make informed decisions together regarding the most suitable anticoagulant for long-term treatment.
Global and local concerns regarding low public awareness of AF and anticoagulants persist.83,84 In Vietnam, public education programmes have been initiated, including the launch of dedicated websites by the Vietnamese Ministry of Health Stroke Free Program, to promote stroke prevention among patients.85 However, there remains a need for more active and widespread implementation of patient education programmes. Addressing this gap through enhanced educational initiatives can significantly contribute to raising awareness and understanding of AF and the role of anticoagulants in its management.
Conclusion
Part 2 of this review provides practical guidance on anticoagulation selection strategies for specific patient populations, including those with renal impairment, diabetes, advanced age, CAD, and individuals with a bioprosthetic valve. The choice of anticoagulation is based on a comprehensive assessment of the unique clinical challenges faced by each patient group, complemented by in-depth reviews of subgroup analyses from pivotal studies and real-world evidence. By considering these factors, appropriate anticoagulation recommendations are proposed to address the specific clinical problems of patients and optimise treatment outcomes.