Non-alcoholic fatty liver disease (NAFLD) is a spectrum of fat-associated liver conditions that can result in end-stage liver disease and the need for liver transplantation. Simple steatosis, or fatty liver, occurs early in NAFLD and may progress to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis with an increased risk of hepatocellular carcinoma. Non-alcoholic liver diseases are associated with the appearance of cardiovascular diseases (CVD). In fact, it has been estimated that CVDs are present in 47% of patients with NAFLD.1
The possible relationship between liver and CVDs has been detailed in recent years. There has been a continuous increase in the prevalence of NAFLD and, in those in whom NAFLD has progressed towards NASH, this is associated with greater age, BMI and triglyceride concentrations. NAFLD has also been verified in patients with diabetes, arterial hypertension or insulin resistance.2 Currently, both NAFLD and NASH are considered to be the hepatic manifestation of the metabolic syndrome, whose relationship with the onset and progression of arteriosclerosis has been demonstrated even at a very early age.3,4 Compared with control subjects who do not have hepatic steatosis, patients with NAFLD have a higher prevalence of atherosclerosis, as demonstrated by increased intimal thickness of the carotid wall, number of atherosclerotic plaques and increased plasma markers of endothelial dysfunction, which are independent of obesity and other established risk factors.5,6 This increase in atherosclerosis in patients with NAFLD has been shown to be associated with alterations in lipid metabolism. In this population, low-density lipoprotein receptors are downregulated, resulting in increased cholesterol uptake and lipoprotein synthesis, increasing liver triglyceride levels.7,8 These changes contribute directly to the development of atherosclerosis and may contribute to generalised plaque formation in the coronary arteries.9,10 In addition, NAFLD is associated with kidney disease, which may be associated with the future risk of cardiac arrhythmia.11
Finally, NAFLD has been identified as a risk factor for early subclinical abnormalities in myocardial metabolism, as well as cardiac structure and function. In particular, NAFLD has been shown to be associated with impaired cardiac energy metabolism, left ventricular hypertrophy and impaired diastolic function. NAFLD has also been shown to contribute to the development of heart failure.12
The result of this association between both NASH and CVD pathologies, and the greater progression of liver disease in NASH, is that all-cause mortality rates are even greater in patients with NASH with heart disorders than in patients with NAFLD without those features.13 All this means that patients with NAFLD have 69% greater mortality from any cause than the general population, in whom mortality is mainly due to CVDs.
In this study, we undertook a systematic review of the relationship between NAFLD and CVDs to summarise the clinical approach and the role of the cardiologist in improving management.
Materials and Methods
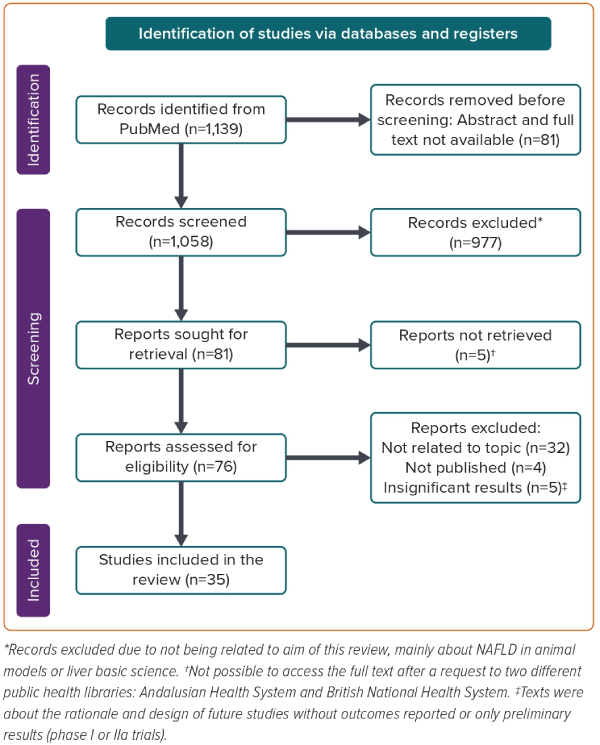
The systematic search was developed using keywords and medical subject headings for cardiology, NAFLD and human model studies ((cardiac[Title/Abstract] OR cardiology[Title/Abstract]) AND (NASH[Title/Abstract] OR “fatty liver disease” [Title/Abstract])) NOT (mice[Title/Abstract] OR mouse[Title/Abstract]), and was conducted in PubMed, from inception until 13 March 2023. Only articles published in English were included. Studies were also included if they were a randomised controlled trial (RCT), systematic review or meta-analysis. Using this search strategy identified 81 articles. After assessing the eligibility of each of these articles, 35 were selected and included in this review (Figure 1).11,12,14–46
Cardiovascular Diseases and Non-alcoholic Fatty Liver Disease
Atherosclerosis
Several studies have reported the association between NAFLD and atherosclerosis independent of traditional risk factors.1,4,8,10 Although atherosclerosis represents a spectrum of disease processes, coronary artery disease (CAD) is the most prevalent. In their meta-analysis including 67,070 patients with NAFLD, Toh et al. reported that the prevalence of coronary heart disease was 44.6%.14 The probability of CAD was higher among NAFLD patients than non-NAFLD patients (OR 1.33; 95% CI [1.21–1.45]; p<0.0001), as well as overall in patients with moderate and severe steatosis compared with those with mild steatosis.1,4,8,10,14 Another meta-analysis of four studies comprising 10,060 patients provided data regarding the association between NAFLD and the progression of coronary artery calcification (OR 1.5; 95% CI [1.34–1.68; p=0.001).15 In a prospective study of 392 patients undergoing coronary angiography, NAFLD was significantly more often diagnosed in patients with than without clinically relevant CAD (75.0% versus 63.1%; p=0.0068).16 Conversely, according to a meta-analysis of 20 studies, patients with NAFLD had a significantly increased risk of MI than patients without NAFLD (OR 1.66; 95% CI [1.39–1.99]).17
Whether the association between NAFLD and CAD is causative or may also be due to shared underlying risk factors remains to be clarified. One European study tried to discover confounding factors by using genetic variants as proxies for the exposure of interest (liver fat content and NAFLD).18 That study analysed a common variant (I148M) in the gene encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3), which is known to be a strong predictor for liver fat content and NAFLD, without being associated with other risk factors for CAD. In that study, the PNPLA3-I148M genotype was associated with increases in liver fat content, NAFLD and liver cirrhosis. However, this genotype was not associated with the risk of CAD. This implies that liver fat content and NAFLD could not be causal factors in the development of CAD, and that the association between NAFLD and CAD may have been influenced by confounding factors, such as age, obesity, low physical activity, smoking, hypertension and plasma lipid levels.18 However, all studies conducted on Asian populations reported a significant independent association between NAFLD and an increased risk of acute coronary syndrome, which may be explained by the different lifestyles and epidemiological characteristics compared with western subjects.19
Compared with non-NAFLD patients, those with NAFLD have been reported to have a higher incidence of cerebrovascular disease. In pooled analysis of 25,839 individuals with NAFLD, the incidence of stroke was 5.04%, with ischaemic stroke being more frequent than haemorrhagic stroke (6.05% versus 2.22%).20 In their meta-analysis, Alon et al. also reported that ischaemic stroke was more frequent in NAFLD patients (OR 1.41; 95% CI [1.29–1.55]) than in non-NAFLD patients.17
Patients with NAFLD also showed a higher prevalence of carotid atherosclerosis: in pooled analysis of 7,951 patients with NAFLD, 35.02% had carotid atherosclerosis and had a greater mean carotid intima–media thickness (CIMT) than those without NAFLD.20 The majority of articles evaluating CIMT supported the finding of elevated CIMT in NAFLD patients compared with patients without liver involvement, as confirmed in the meta-analysis of Pacifico et al.21
Considering all these findings, regardless of the presence of confounding factors, NAFLD should be considered as a potential cardiovascular risk-enhancing factor that increases pre-test probability of CAD and cerebrovascular disease. This may help identify individuals who would benefit from more conscientious risk factor management.
Heart Failure
NAFLD has been associated with cardiac remodelling, leading to the appearance of heart failure. In their meta-analysis, Alon et al. reported a higher incidence of heart failure in NAFLD patients (OR 1.62; 95% CI [1.43–1.84]).17 Another systematic review of four studies with 924 children evaluating the association between NAFLD and abnormalities in cardiac structure indicated that youths with NAFLD have increased hallmarks of diastolic left ventricular dysfunction as well as left ventricular hypertrophy.21
The results of a recent meta-analysis of >1.4 million subjects showed that patients with NAFLD have a 60% risk of incident heart failure.22 That meta-analysis could not establish a relationship with the different categories of heart failure, because it included only one study that reported the association between NAFLD and incident heart failure based on left ventricular ejection fraction (LVEF). That single study showed a significantly increased risk of heart failure with preserved ejection fraction.22 The mechanisms underlying the relationship between diastolic left ventricular dysfunction and NAFLD are not completely clear, but oxidative stress, inflammation and insulin resistance promoted by NAFLD may contribute to the development of heart failure, particularly heart failure with preserved ejection fraction.17
Cardiac Arrhythmias
AF is probably the arrhythmia most related to NAFLD in the literature. In the meta-analysis of Alon et al., patients with NAFLD showed a significantly increased risk of AF compared with non-NAFLD patients (OR 1.27; 95% CI [1.18–1.37]).17 This was also confirmed in a systematic review that included 401,745 individuals and was independent of common confounding factors.23
A meta-analysis by Gong et al., which included 19 observational studies with 7,012,960 patients, found that NAFLD was significantly associated with an approximately twofold higher risk of AF (OR 1.71; 95% CI [1.14–2.57]).11 NAFLD was not only associated with AF, but also with a prolonged QT interval (OR 2.86; 95% CI [1.64–4.99]) and premature atrial/ventricular contraction (OR 2.53; 95% CI [1.70–3.78]).11
Other systematic reviews have confirmed the relationship between NAFLD and both premature ventricular contractions and prolongation of the QTc interval.23 A meta-analysis that included 3,651 patients found an approximately fivefold greater risk of cardiac conduction defects among patients with that without NAFLD (OR 5.17; 95% CI [1.34–20.01]). These conduction defects included atrioventricular node block, bundle branch block and fascicular block.24 Heart block was also analysed by Gong et al. in their meta-analysis of generated, with a reported pooled OR for participants with versus without NAFLD of 2.65 (95% CI [1.88–3.72]).11
The reasons behind this relationship are not completely understood but are likely related to an increased level of inflammatory cytokines (Interleukin [IL]-1, IL-6, Transforming growth factor-β), which are arrhythmogenic. Some of these cytokines result in cardiac fibrosis and cardiac myocyte apoptosis during the onset and progression of NAFLD, leading to changes in the electrophysiological properties and structure of the myocardium.11,24
Chronic Kidney Disease
NAFLD has been suggested to play a potential pathogenic role in the occurrence of renal dysfunction. In the meta-analysis of Pacifico et al., NAFLD was associated with an increased risk of reduced estimated glomerular filtration rate and/or microalbuminuria (OR 2.54; 95% CI [1.16–5.57]; p<0.05).21 Awareness of subclinical renal dysfunction related to NAFLD is crucial to identify in order for treatment to start in the early phases to avoid the development of chronic kidney disease.21
Echocardiography Assessment and Non-alcoholic Fatty Liver Disease
Current guidelines emphasise the importance of thorough cardiovascular screening in patients with NAFLD.25,26 This cardiovascular screening has focused on the study of cardiovascular risk factors, with stress echocardiography having a role in the non-invasive diagnosis of myocardial ischaemia in patients with NASH cirrhosis before liver transplantation.25 Nevertheless, several recent studies have demonstrated the association between abnormalities in systolic and diastolic cardiac function parameters and NAFLD.25,26 Therefore, cardiac imaging techniques, especially echocardiography, are becoming relevant in the cardiovascular screening of patients with NAFLD.
Regarding systolic cardiac function, in a meta-analysis including 14 studies assessing the association between NAFLD and LVEF, Borges-Canha et al. found no significant difference in LVEF between NAFLD and non-NAFLD populations.27 However, in a more recent meta-analysis, Yong et al. found that NAFLD patients had worse systolic indices with lower LVEF than non-NAFLD patients.28 In another meta-analysis evaluating subclinical cardiac damage in patients with biopsy-proven NAFLD, Oikonomidou et al. found that global longitudinal strain (GLS) was significantly lower in NAFLD patients than in controls.29 Furthermore, in a prospective study, van Wagner et al. reported that patients with NAFLD had a greater reduction in left ventricle systolic function (GLS and LVEF) than patients without NAFLD, demonstrating that NAFLD is potentially associated with greater progression of subclinical left ventricle systolic dysfunction independent of heart failure risk factors.30 Based on the most recent literature, NAFLD seems to be associated with worsening LVEF and GLS, but more studies, ideally prospective studies, are needed to confirm these findings.
With regard to diastolic function, it seems clear that there is a significant association between NAFLD and diastolic function parameters, specifically lower E/A and higher E/e′.12,27,28,30 These findings have also been described in a population with type 2 diabetes.31 Furthermore, the meta-analysis by Wijarnpreecha et al. found that patients with NAFLD had approximately twofold increased odds of having diastolic cardiac dysfunction than individuals without NAFLD.32 Furthermore, in addition to describing an association between NAFLD and diastolic function parameters, a subgroup analysis of cardiac function based on the severity of steatosis in the meta-analysis of Yong et al. revealed that the severity of liver pathology correlated more with diastolic dysfunction than with systolic function; specifically, those with moderate–severe steatosis had a significantly larger decrease in E/A that those with mild steatosis, but there was no significant difference in LVEF between the two groups.28
Epicardial fat thickness (EFT) is another parameter whose association with NAFLD has recently been studied. In their respective meta-analyses, Oikonomidou et al., Yong et al. and Orci et al. described high EFT in patients with NAFLD compared with controls.28,29,33 Furthermore, Orci et al. reported that EFT was significantly higher in patients with severe liver steatosis than in patients with mild–moderate liver steatosis, concluding that EFT may represent a useful surrogate for assessing the presence and severity of NAFLD in a non-invasive manner.33
Considering all of the above, we consider that when NAFLD is diagnosed, routine echocardiography should be performed to assess cardiac structure and function.
Management of Non-alcoholic Fatty Liver Disease
Dietary Patterns
NAFLD is predominantly managed by lifestyle interventions. Dietary patterns have a crucial role in the management of NAFLD and may help with weight loss and modify the accumulation of free fatty acids and triglycerides in the liver. The consumption of fibre, as a healthy eating habit with a beneficial effect on NAFLD, is supported by results from the RESMENA trial (n=70). In that trial, participants with higher consumption of insoluble fibre (>7.5 g/day) showed improvements in the fatty liver index, hepatic steatosis index and NAFLD liver fat score.34 Consumption of >8.8 g/day fruit fibre resulted in significant improvements in transaminases.34
Several studies have evaluated the Mediterranean diet, which is usually recommended for NAFLD. In the MEDINA RCT (n=42), after 12 weeks, significant improvements were seen in intrahepatic lipids in the low-fat diet group (−17% [log scale]; p=0.02), but not in the Mediterranean diet group (−8%; p=0.069).35 In addition, insulin resistance, as evaluated using Homeostatic Model Assessment, decreased in the low-fat diet group (from 6.5 ± 5.6 to 5.5 ± 5.5 [mean ± SD]; p<0.01), but not in the Mediterranean diet group (from 4.4 ± 3.2 to 3.9 ± 2.3 [mean ± SD]; p=0.07).35 Nonetheless, visceral fat, evaluated using bioelectrical impedance analysis, decreased significantly in both the low-fat diet and Mediterranean diet groups (−76% [log scale; p≤0.0005] and −61% [p≤0.0005], respectively).35
The Fatty Liver in Obesity (FLiO) RCT (n=98) evaluated the effects of the Mediterranean diet at 2 years compared with a control diet, which was based on American Heart Association (AHA) guidelines that suggest three to five meals per day, with a balanced distribution of macronutrients (50–55% of total caloric value from carbohydrates, 15% from proteins and 30% from lipids) and a healthy fatty acid profile.36 Conversely, the FLiO diet, based on the Mediterranean diet, was designed with a higher meal frequency (seven meals per day): breakfast, lunch, dinner, two snacks in the morning and two snacks in the afternoon. The established macronutrient distribution was 40–45% of the total caloric value from carbohydrates (low glycaemic index), 25% from proteins (mainly from vegetable sources) and 30–35% from lipids (extra virgin olive oil and Ω-3 fatty acids to the detriment of saturated and trans fats). Both the AHA and FLiO diets significantly reduced body weight at 6 months (−9.7% versus −10.1%), 12 months (−6.7% versus −9.6%) and 24 months (−4.8% versus −7.6%), with significant improvements in body composition and biochemical and liver determinations throughout the intervention. At the end of the follow-up period, the FLiO group had a greater decrease in transaminases, liver stiffness and Fatty Liver Index.36
A recent systematic review that included a previous study and data from three RCTs in patients with NAFLD but without diabetes showed there was moderate evidence that a low-carbohydrate (versus a low-calorie diet) and the Mediterranean diet (versus a low-fat, high-carbohydrate diet) result in greater reductions in hepatic fat content (−27% [p=0.008, one study, n=18] and −4.4% [p=0.030, one study, n=12], respectively).37 These studies support the potential benefits of the Mediterranean diet in NAFLD, but large RCTs are needed to determine the impact of this dietary pattern on hospitalisations and mortality.
Exercise Training
Exercise training is the other pillar of lifestyle intervention to improve weight loss in NAFLD. The effect of high-intensity interval training (HIIT) on liver fat and cardiac function was studied in one RCT (n=33).38 Patients with NAFLD were assigned to 12 weeks HIIT, which involved thrice-weekly cycle ergometry for 30–40 minutes, or standard care. HIIT decreased liver fat (from 11 ± 5% to 8 ± 2% versus from 10 ± 4% to 10 ± 4% [mean ± SD] in the control group; p=0.019), whole-body fat mass (from 35 ± 7 to 33 ± 8 kg versus from 31 ± 9 to 32 ± 9 kg [mean ± SD]; p=0.013), alanine aminotransferase (ALT; from 52 ± 29 to 42 ± 20 versus from 47 ± 22 to 51 ± 24 units/l [mean ± SD]; p=0.016) and aspartate aminotransferase (AST; from 36 ± 18 to 33 ± 15 versus from 31 ± 8 to 35 ± 8 units/l [mean ± SD]; p=0.017) and increased the early diastolic filling rate (from 244 ± 84 to 302 ± 107 ml/s versus from 255 ± 82 to 251 ± 82 ml/s [mean ± SD]; p=0.018).38
This intervention effect was included in a meta-analysis (17 RCTs; n=1,627), evaluating four exercise modalities in patients with NAFLD: HIIT (n=53), aerobic training (AT; n=554), resistance training (RT; n=232), AT+RT (n=100) and control (population with diet intervention or routine drug treatment without any of these four exercise modalities explained: HIIT, AT, RT or AT + RT; n=668).39 The results of the statistical analysis showed that the AT+RT exercise intervention had the most significant effect on the mean difference in total serum cholesterol. ALT and AST levels improved, but there were no significant differences among the four exercise intervention groups.39 This evidence supports the benefits of AT+RT and HIIT in patients with NAFLD who can tolerate these exercise programmes.
Pharmacological Management
Currently, no approved medications are listed for NAFLD and its associated fibrosis. Due to the association of NAFLD with metabolic syndrome (obesity, type 2 diabetes [T2D] and dyslipidaemia), there are many drugs with potential benefits undergoing clinical studies.
Statins, the cornerstone of management for dyslipidaemia, are safe and can improve liver tests and reduce cardiovascular morbidity in patients with mild to moderately abnormal liver tests that are potentially attributable to NAFLD.40 Reducing the synthesis of triglycerides could also be an efficacious strategy for the NAFLD population, but novel therapies using antisense oligonucleotides in this pathophysiology require further investigation.41
NAFLD is associated with insulin resistance and T2D. Antidiabetic drugs that increase insulin sensitivity are associated with a reduced risk of CVD and may have beneficial effects in the progression of NAFLD.42 The effects of thiazolidinediones on advanced liver fibrosis were studied in a meta-analysis (eight RCTs; n=516).43 Pioglitazone was associated with improved advanced fibrosis (OR 3.15; 95% CI [1.25–7.93]; p=0.01; I2=0%), fibrosis of any stage (OR 1.66; 95% CI [1.12–2.47]; p=0.01; I2=0%) and NASH resolution (OR 3.22; 95% CI [2.17–4.79]; p<0.001; I2=0%).43 However, weight gain and lower limb oedema occurred more frequently with thiazolidinedione therapy (initial body weight+2.70%; 95% CI [1.96–4.34%]; p=0.001).43
A recent systematic review has also reported potential benefits of empagliflozin, a sodium–glucose cotransporter 2 (SGLT2) inhibitor with beneficial effects on T2D, heart failure and chronic kidney disease in individuals with NAFLD, improving hepatic triglycerides, decreasing visceral fat and reducing inflammatory markers and insulin resistance.44
Glucagon-like peptide-1 (GLP-1) analogues have a multitarget beneficial action in individuals with T2D, lowering plasma glucose, reducing liver steatosis and inflammation, improving cardiac function and protecting against kidney dysfunction.45 Large retrospective studies on individuals with T2D and small Phase II RCTs in individuals with NAFLD and obesity treated with the GLP-1 analogue liraglutide showed significant dose-dependent increases in insulin sensitivity, decreases in liver enzymes and advanced liver fibrosis, and amelioration of advanced liver fibrosis.45
A Phase II RCT showed the efficacy of semaglutide 0.4 mg on the resolution of NAFLD without worsening fibrosis, but it may not improve fibrosis.46 On average, GLP-1 agonists can result in weight loss of 4–5 kg, which continues up to week 44 of the regimen.46 Because current clinical management consists of lifestyle changes leading to weight loss, GLP-1 agonists will likely play a greater role in the treatment of patients with NAFLD in the near future.
Conclusion
NAFLD is considered to be the liver manifestation of metabolic syndrome. NAFLD increases the risk of developing several atherosclerotic CVDs, such as CAD or ischaemic stroke, but it is also involved in heart failure with preserved ejection fraction or AF.
When NAFLD is diagnosed and the patient is referred to a cardiologist, routine echocardiography should be performed, even in the case of asymptomatic patients, to screen out early subclinical abnormalities, mainly diastolic function parameters and EFT. Clinical management is based on lifestyle changes with the aim of weight loss, with the current approach based on the Mediterranean diet and intense exercise training.
Despite the dearth of approved drugs for NAFLD, new potential treatments with clinical benefits, such as GLP-1 agonists or SGLT2 inhibitors, may help us to face this issue in the coming years. 
Clinical Perspective
- Non-alcoholic fatty liver disease (NAFLD) is associated with metabolic syndrome and an increased risk of cardiovascular diseases: CAD, heart failure, electrical disorders.
- Several echocardiographic values, such as cardiac diastolic impairment or epicardial fat thickness, are related to NAFLD progression.
- NAFLD is predominantly managed by lifestyle changes. The Mediterranean diet and aerobic plus resistance training or high-intensity interval training improve determinants of liver function and body composition, and lead to weight loss.
- Although there are no approved treatments for NAFLD or its associated fibrosis, potential drugs such as GLP-1 agonists could play a greater role in the treatment of NAFLD in the near future.