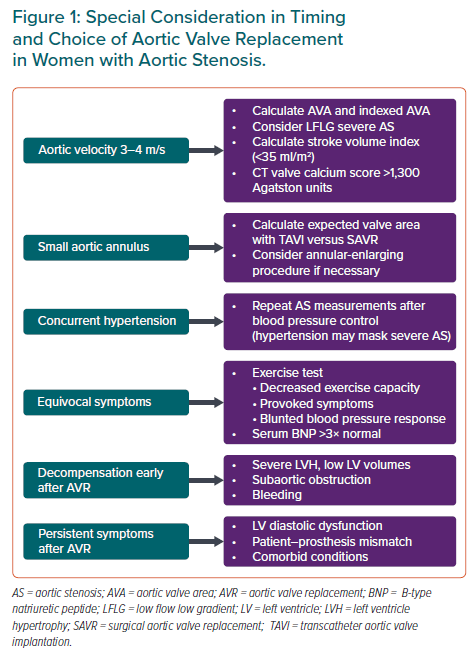
In adults with severe aortic stenosis (AS) there are two steps in the decision-making process. First, is there an indication for aortic valve replacement (AVR)? Second, what type of valve replacement is most appropriate in this patient? Both European Society of Cardiology/European Association of Cardio-Thoracic Surgery (ESC/EACTS) and American College of Cardiology/American Heart Association (ACC/AHA) guidelines strongly recommend AVR in symptomatic adults with severe AS, regardless of sex or age, unless life expectancy or quality of life after intervention would be so poor that palliative care is more appropriate.1,2 Indications for AVR in asymptomatic adults with severe AS include left ventricular systolic dysfunction (ejection fraction <50%), an abnormal exercise stress test or markedly elevated serum B-type natriuretic peptide (BNP) levels, very severe AS (maximum velocity >5 m/s), rapid haemodynamic progression or severe valve calcification. Based on the current published evidence base, current recommendations for timing of AVR apply to all adult patients, regardless of age or sex, with sparse data on racial/ethnic differences as well.3 Even so, differences in the clinical presentation and diagnosis of severe AS in women and in older adults do affect clinical decision making about the timing of AVR in each patient (Figure 1).4
Sex-specific Factors in Timing of Valve Intervention
Importantly, there are sex-specific differences in women compared to men with AS, both on multimodality imaging and for grading of AS severity.5,6 For example, older women are more likely to have paradoxical low-flow low-gradient severe AS with a normal ejection fraction and thus the diagnosis of severe AS may be missed. Often, these women are referred later in the disease course or not referred at all, even though valve replacement is indicated. In addition, although women typically are more symptomatic than men and more likely to experience shortness of breath and light-headedness, symptoms may erroneously be attributed to comorbid conditions or ageing, leading to delays in intervention.7 For these reasons, it is prudent to consider exercise testing or obtaining a serum BNP level in older women with echocardiographic evidence of moderate or severe AS for an objective measure of symptom status. Measurement of the severity of leaflet calcification by CT also may be helpful, using sex-specific standards for severe calcification, given that women have less calcification and more leaflet fibrosis than men.8–10
Choice of Valve Intervention
In a patient with an indication for AVR, the next step is to determine the most appropriate type of valve intervention in that patient. The first consideration is the choice between a mechanical valve, which is durable long-term but requires lifelong anticoagulation, versus a bioprosthetic valve, which is less durable but does not require anticoagulation. If a bioprosthetic valve is appropriate, the next step is to decide between surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI).11
Current ESC/EACTS and ACC/AHA recommendations do consider age (as a surrogate for life expectancy) in this decision process, but there are not enough data to support sex-specific recommendations at this time. Even so, from a clinical point of view, there are several age- and sex-related issues to be considered in each patient in a shared clinical decision-making process.
Choice of Prosthetic Valve Type
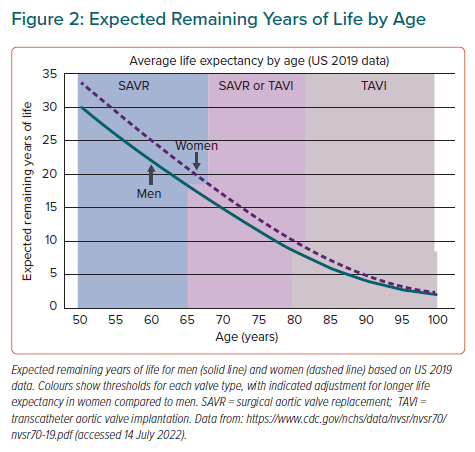
In younger patients with a long life expectancy, a mechanical prosthetic valve offers long-term durability with low rates of valve dysfunction or need for repeat intervention over the patient’s lifetime with an acceptable risk of bleeding and thrombotic events due to the need for continuous vitamin-K antagonist (VKA) anticoagulation. Mechanical aortic valve replacement is considered reasonable for severe symptomatic AS in patients aged <60 years in the ESC/EACTS guidelines. The ACC/AHA guidelines suggest mechanical valve replacement for patients aged <50 years, with either a mechanical or bioprosthetic valve being reasonable in those aged between 50 and 65 years. However, considering the longer life-expectancy in women versus men at any given age, the age threshold for considering a bioprosthetic valve probably should be about 3 years older in women than in men (Figure 2).
Exceptions to these recommendations are patients who have comorbid conditions that preclude or increase the risk of long-term anticoagulation; in these patients a bioprosthetic valve is reasonable. Younger women who desire future pregnancy also are a special situation given the high maternal risk of valve thrombosis during pregnancy and the risk to the foetus of VKA anticoagulation. Younger women may choose a bioprosthetic valve, despite the need for a subsequent intervention to avoid the risks of a mechanical valve and anticoagulation during pregnancy.
In some younger patients, additional options such as a valve-sparing root replacement or a pulmonic valve autograft also might be considered.
Surgical Versus Transcatheter Approach
TAVI now is established as a standard treatment for severe AS, equivalent or better than SAVR in terms of immediate hemodynamic results and short and mid-term mortality, based on a series of randomised controlled clinical trials (RCTs) in patients at high-, intermediate- or low-estimated surgical risk.12–15 In the low-risk PARTNER 3 trial of patients with severe AS and a mean age of 73 years, overall mortality was lower with TAVI (TAVI, 11.5%, SAVR, 17.4%; HR 0.63; 95% CI [ 0.45 to 0.88]; p=0.007) at 2 years follow up.16 TAVI was associated with lower incidence of disabling stroke at 30 days and new onset AF with no significant difference between groups in major vascular complications, new permanent pacemaker implantation and moderate or severe paravalvular regurgitation. The benefits of TAVI are greatest in older patients; although relative risk is similar across the ages included in these studies, the absolute risk of mortality and stroke is higher in older adults, so the same relative reduction is a greater absolute reduction in risk.
Rates of valve deterioration and function have been low in the older adults included in these clinical trials, but robust data on valve durability are only available out to 5 years. TAVI valve durability in younger patients or at longer term follow-up has not yet been established. Both prosthesis (valvular shear stress, prosthetic size and calcification) and patient-related factors (sex, obesity) have been identified as predictors of valve deterioration.17 Thus, the primary considerations in choosing between SAVR and transfemoral TAVI in adults undergoing bioprosthetic aortic valve replacement are expected patient longevity; the ESC/EACTS guidelines also continue to consider surgical risk in decision making although surgical risk (other than prohibitive) is not a consideration in the ACC/AHA guidelines.
For patients with a prohibitive surgical risk and a post-procedure life expectancy >1 year with an acceptable quality of life and suitable valve and vascular anatomy, both the ACC/AHA and ESC/EACTS guidelines recommend TAVI. TAVI also is recommended in patients at high surgical risk (>8%) or those aged >75 years (ESC/EACTS guidelines) or >80 years (ACC/AHA guidelines) regardless of surgical risk. In both guidelines, age is used as a surrogate for life expectancy given the average life expectancy at age 75 to 80 years is approximately 10 years.1,2 However, if age is used as a surrogate for life expectancy, the threshold for recommending TAVI in all women should be a few years older in women than men because women have more expected remaining years of life than men at any given age (Figure 2).18
Decision making becomes more complicated in patients aged <75 to 80 years. The ACC/AHA guidelines recommend either SAVR or TAVI in patients aged 65–70 years, based on a Heart Valve Team assessment and shared decision making taking into account the patient’s life expectancy and valve durability but also patient’s preferences along with anatomical and procedural factors.9 In contrast, the ESC/EACTS guidelines consider SAVR and TAVI equivalent in patients aged <75 years only in patients with an intermediate surgical risk (4–8%), instead recommending SAVR when surgical risk is low (<4%). These differences in recommendations in the age group between 65 and 75 years reflect the scarcity of data on long term TAVI valve durability.
Similarly, in the ACC/AHA guidelines, SAVR remains the preferred choice in patients aged <65 years given an expected patient longevity >20 years and unknown long-term transcatheter valve durability. The surgical approach remains the preferred approach even in older patients with concomitant multivessel coronary, bicuspid valve disease, low coronary height, severe annular or subaortic calcification, or unfavourable peripheral vascular anatomy that precludes a transfemoral approach.
Sex-specific Clinical and Anatomic Factors Impacting Choice of Intervention
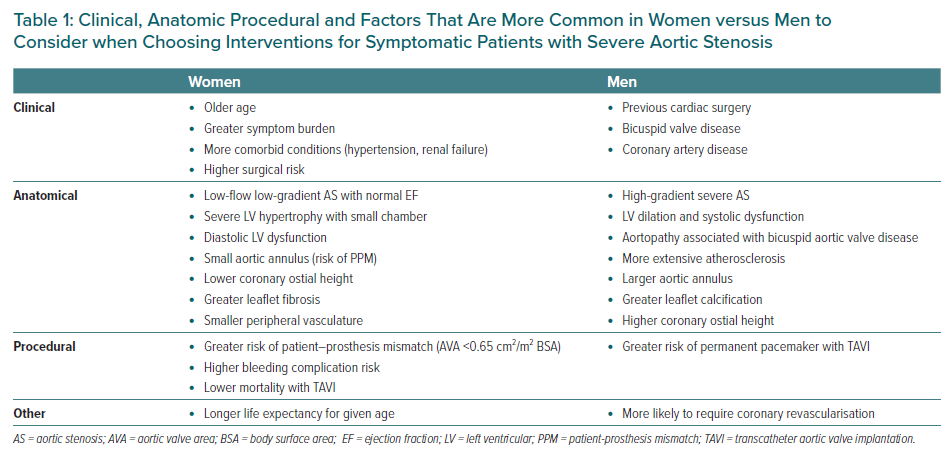
Several clinical and anatomic differences between men and women affect the choice of SAVR versus TAVI (Table 1). Women are approximately 3 years older at the time of presentation, with a higher prevalence of comorbidities such as hypertension and renal failure than men but lower cardiovascular comorbidities such as coronary artery disease and AF.7,10 Men, on the other hand, more often have a history of previous cardiac surgery and reduced left ventricular ejection fraction.7 Men are more likely to have bicuspid aortic valve disease with concurrent dilation of the aortic sinuses and ascending aorta, often necessitating a combined SAVR and graft replacement of the aorta. There is also higher prevalence of coronary artery disease in men, favouring SAVR and coronary artery bypass grafting or a hybrid approach with TAVI and percutaneous coronary intervention.19
From an anatomical point-of-view, the aortic annulus typically is smaller in women compared to men. Smaller prosthetic valves in women result in higher incidence of patient–prosthesis mismatch after SAVR or TAVI in women.20–22 For any given valve annulus diameter, a TAVI valve has a larger effective orifice area and lower transvalvular gradient than the same size SAVR valve because of the smaller supporting structure of the TAVI valve. On the other hand, with a very small annulus, surgery allows the option of an annular-enlarging procedure and placement of a larger valve size, whereas the size of the TAVI valve is limited to the size of the annulus. However, there are no sex-specific data on outcomes with SAVR and root-enlarging procedures. Some newer surgical valves with designs similar to a TAVI valve also offer improved valve haemodynamics. In addition, the distance from the aortic valve plane to the coronary ostium also may be shorter in women, which may limit the TAVI approach.
The size of peripheral arteries is smaller in women than in men, in part related to a smaller body size. This may affect femoral artery access for TAVI and increase the risk of vascular complications. Current guidelines only recommend transfemoral TAVI, not alternative access routes, so that SAVR should be considered when vascular access via a transfemoral route is not possible.
Left ventricular anatomical differences between men and women also affect procedural risk and long-term outcomes. Women tend to have small, hypertrophied ventricles with severe diastolic dysfunction. Reduced left ventricular volumes during the procedure may result in a low forward stroke volume and hypotension or cardiogenic shock. After relief of outflow obstruction, the left ventricle may become hyperdynamic with small ventricular volumes, again precipitating a low cardiac output and hypotension. Management can be challenging as it is difficult to maintain adequate left ventricular volumes without an excessive rise in left atrial pressure. Left ventricular diastolic dysfunction often worsens after TAVI or SAVR because left ventricular myocardial hypertrophy regresses more quickly than myocardial fibrosis, resulting in higher ratio of fibrosis to myocardium and increased chamber stiffness. In contrast, men are more likely to have left ventricular dilation and systolic dysfunction. If left ventricular dysfunction was due to high afterload from valve obstruction, an improvement in left ventricular ejection fraction occurs early after TAVI or SAVR.
Impact of Age and Sex on Procedural Complications
Data from the Society of Thoracic Surgeons/ACC Transcatheter Valve Therapy Registry between 2011 and 2014 showed that 1-year mortality was lower in women versus men with AS undergoing TAVI (21.3% versus 24.5%; adjusted HR 0.73; 95% CI [0.63–0.85]; p<0.001), despite women being older and having higher rates of peri-procedural vascular complications, bleeding events and stroke.23
In a meta-analysis of RCTs prior to 2016 that compared TAVI (n=1,898) to SAVR (n=1,908), although TAVI was associated with a 13% relative risk reduction in all-cause death overall, subgroup analysis showed the benefit of TAVI was seen in women (HR 0.68, 95% CI [0.50–0.91]; p=0.010) but not men (0.99 [0.77–1.28]; p=0.952).13 These findings were confirmed in a separate meta-analysis – with many of the same studies included – showing that women have a 26–31% lower mortality odds with TAVI compared to SAVR whereas no difference was seen in men.24 However, in a multicentre registry of 1,159 men and 1,370 women who underwent TAVI for severe AS between 2008 and 2016, sex differences in mortality that were evident from 2008 to 2012 were no longer seen after 2013, most likely related to technological improvements and better valve sizing.25 However, more recent data from 2012–2018 with 188,325 hospitalisations for TAVI in the National Inpatient Sample data found higher mortality in women aged 81–90 years undergoing TAVI compared to men (3.0% versus 2.1%; p<0.01).26
Major Bleeding
Vascular complications and major bleeding tend to be more common in women than men undergoing TAVI.25,26 Vascular complications and bleeding are associated with a greater sheath-to-femoral ratio as well as lower body mass, smaller iliofemoral arteries and greater vascular tortuosity in women.27–29 Therefore, appropriate imaging of the iliofemoral vasculature and consideration of the vascular access route and risk is particularly important in women. These findings were confirmed in a patient-level meta-analysis of RCTs and registry data, including over 11,000 patients undergoing TAVI, which showed a higher rate of vascular complications (6.3% versus 3.4%; p<0.001) and major bleeding (10.5% versus 8.5%; p=0.003) in women than men, regardless of TAVI valve type.30
Stroke
Based on the meta-analysis of RCTs and registry data from before 2015, there appears to be a slightly higher risk of stroke in women undergoing TAVI compared to men (4.4% versus 3.6%; p=0.029).30 However, the more relevant comparison is the risk of stroke with SAVR versus TAVI which does not appear to be different in women compared to men.
Permanent Pacemaker
Both men and women are at risk of needing a permanent pacemaker for heart block after either SAVR or TAVI because of the close proximity of the conduction system and the aortic annulus, but the risk with SAVR appears to be lower than the risk with TAVI. The long-term consequences of a permanent pacemaker include pacing-induced ventricular dysfunction, risk of device or lead infection and the risk of tricuspid regurgitation related to the position of the lead across the tricuspid valve. Although some of these long-term adverse effects might be reduced with use of leadless pacemakers, all these factors should be considered in decisions about valve type. The rate of permanent pacemaker implantation after TAVI is quite variable in published studies, ranging from approximately 4% to 20%. In a meta-analysis of 78 studies with over 30,000 patients undergoing TAVI, predictors of the need for a permanent pacemaker included male sex, baseline or post-procedural conduction abnormalities and use of a self-expandable valve.31 A second meta-analysis confirmed a lower rate of permanent pacemaker insertion in women, compared to men, after a balloon-expandable TAVI.32 In contrast, the risk of needing a permanent pacemaker after SAVR is approximately 4%.33
Paravalvular Regurgitation
Paravalvular regurgitation has become less of an issue in both men and women undergoing TAVI as technical improvements in valve design and use of advanced imaging to optimise valve sizing have reduced the risk of this complication.
Integrative Approaches to Clinical Decision Making
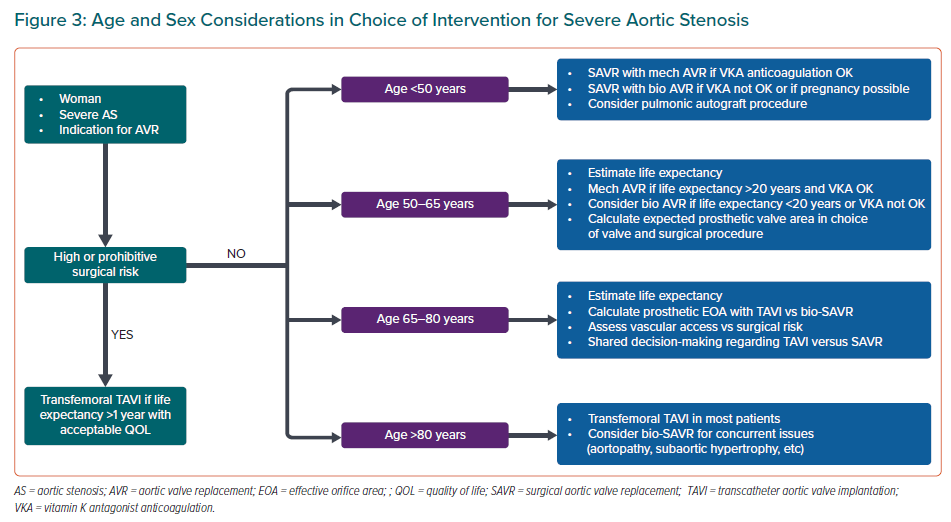
Clinical decision making in adults with AS starts with the recommendations in the ACC/AHA and ESC guidelines. From that starting point, therapy is individualised with consideration of age and sex – as well as other clinical factors – in conjunction with shared decision making, taking patient preferences and values into consideration. We suggest an iterative approach to the choice between TAVI and SAVR in patients who are not at high or prohibitive surgical risk (Figure 3). Our first step is to consider life expectancy, using age as a surrogate but adjusted for sex and other clinical factors. In younger patients, surgical options that avoid a prosthetic valve are appealing in both sexes; in young women avoiding VKA anticoagulation is recommended if future pregnancy is desired. Mechanical AVR remains an appropriate option when life expectancy is >20 years and the patient is able and willing to be on VKA anticoagulation. In patients aged >80 years (or with a life expectancy <10 years for other reasons), TAVI is a reasonable choice. In the intermediate age range of 65–80 years, sex-related differences in outcomes, valve sizing and complications after TAVI versus SAVR become more important, along with the recognition that women have a longer life expectancy at any age compared to men. The timing of AVR for AS and the choice of valve type are complex decisions, best made in the context of a Heart Valve Team at a comprehensive Heart Valve Centre.34 Research is on-going to better define the optimal timing of intervention and to better understand age, sex and racial/ethnic differences in complication rates and long-term outcomes.