Measurement of Blood Pressure
The 2018 European Guidelines on the management of hypertension recommend that the diagnosis of hypertension should not only be based on office blood pressure (BP) but also on out-of-office measurements such as ambulatory or home BP monitoring.1 These recommendations were directed to enable more accurate diagnosis, particularly in the context of white-coat and masked hypertension. A landmark registry-based study performed in Spain that included 63,910 adults recruited from 2004 through 2014 comprising both clinic and 24-hour ambulatory BP measurements provided very strong evidence.2 Follow-up was 4.7 years and 24-hour systolic BP was more strongly associated with all-cause mortality (HR 1.58 per 1-SD increase in BP; 95% CI [1.56–1.60]) than clinic systolic BP (HR 1.02; 95% CI [1.00–1.04]). Corresponding HRs per 1-SD increase in BP were 1.55 (95% CI [1.53–1.57]) for night-time ambulatory systolic BP and 1.54 (95% CI [1.52–1.56]) for daytime ambulatory systolic BP. These relationships were consistent across subgroups of age and sex as well as obesity, diabetes, cardiovascular disease and antihypertensive treatment status. One of the most interesting and revealing results was that masked hypertension was more strongly associated with all-cause mortality (HR 2.83; 95% CI [2.12–3.79]) than sustained hypertension (HR 1.80; 95% CI [1.41–2.31]) or white-coat hypertension (HR 1.79; 95% CI [1.38–2.32]). Similar results were observed for cardiovascular mortality.
The reliability and prognostic value of office, ambulatory and home BP and their associations with left ventricular mass index in untreated subjects were analysed in the IDH trial.3 The study enrolled 408 participants without cardiovascular disease who had their office BP assessed at three visits and completed 3 weeks of home BP measurements (measured twice in the morning and twice in the evening), two 24-hour ambulatory BP recordings, and a 2D echocardiogram. For systolic and diastolic BP, the highest reliability was for home BP, followed by office BP and 24-hour ambulatory BP. Likewise, the strongest correlation with elevated left ventricle mass, as hypertension-mediated organ damage, was found for home BP. This evidence supports the use of out-of-office methods for the diagnosis of various phenotypes of hypertension, such as white-coat and masked hypertension, with a significant and independent prognostic value.
Intensive Blood Pressure Control
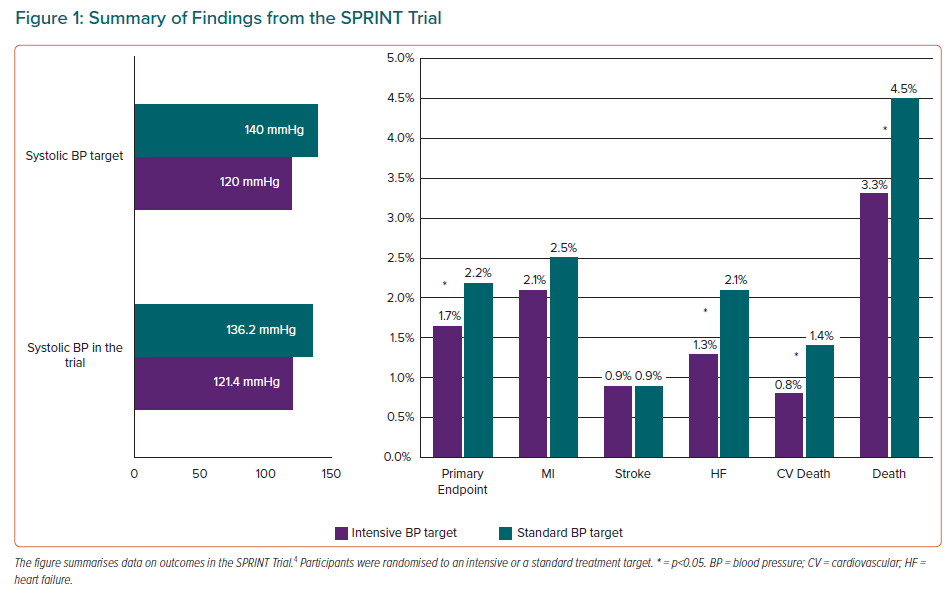
Evidence on intensive BP control has changed substantially in recent years, mainly because of the ground-breaking results of the SPRINT trial.4 This trial recruited 9,361 adults aged ≥50 years who were at increased risk for cardiovascular disease and had an average systolic BP of 130–180 mmHg, without diabetes, previous stroke or formal contraindications to BP lowering beyond 140/90 mmHg. Participants were randomised to an intensive treatment target (systolic BP <120 mmHg) or a standard treatment target (systolic BP <140 mmHg). The primary outcome was a composite of MI, stroke, acute coronary syndrome, acute decompensated heart failure and cardiovascular death. The primary outcome and all-cause mortality were 25% (p<0.001) and 27% (p=0.003) lower in the intensive compared with standard group but no significant reductions in stroke or MI were observed (Figure 1); results also showed a 43% reduction (p=0.005) for cardiovascular death and 38% reduction in acute heart failure (p=0.002) in the intensive treatment group. This benefit in the reduction of cardiovascular events in the intensive treatment group led to the trial being stopped early.
The final report of the SPRINT trial concluded that lower rates of major adverse cardiovascular events (MACE) and lower all-cause mortality were obtained for a systolic BP of <120 mmHg, even after excluding acute heart failure from the primary outcome.5 The reduction in primary outcome in the intensive-treatment group was reproduced in all the prespecified subgroups. Interestingly, in the 2,636 non-institutionalised participants who were aged ≥75 years at baseline, the benefits were similar, resulting in prevention of primary outcome events and all-cause mortality for one in every 28 and 41 participants in the intensive treatment arm, respectively.6 Despite previous concerns about the potential for adverse cognitive effects, intensive BP treatment resulted in a significant reduction in mild cognitive impairment during the trial and a composite of mild cognitive impairment and probable dementia during combined trial and post-trial follow-up.7 Finally, serious adverse events that were specified in the trial protocol and procedure manual, particularly those related to hypotension, syncope, falls and acute kidney injury, were significantly more common during intensive treatment but did not lead to an overall increase in major morbidity or mortality.5
In conclusion, the SPRINT results indicate that more intensive BP reduction provides substantial health benefits that outweigh the risks of adverse events. This is also supported by two meta-analyses.8,9 However, it does not solve the puzzle of what the optimal BP goal is, because the method used for office BP measurement in the SPRINT study (automated measurement without personnel present) had not previously been used in any clinical trial. The ability to generalise the SPRINT results to clinical practice requires accurate assessment of BP and evidence of high cardiovascular risk. These requirements are common to generalisation of other landmark BP treatment trials.
An analysis of six trials (original data from two trials and reconstructed data from four trials) that included 27,414 participants demonstrated that intensive BP treatment with a systolic BP target <140 mmHg was significantly associated with a 21% reduction in MACE (HR 0.79; 95% CI [0.71–0.88]; p<0.001).10 On average, 9.1 months were needed to prevent one MACE per 500 patients with the intensive BP treatment; in contrast, 19.1 and 34.4 months were estimated to avoid one MACE per 200 and 100 patients with standard treatment, respectively.
Therapeutic Approaches
Fixed-dose Combinations
Fixed-dose combinations have been demonstrated to increase adherence and, as a consequence, better BP control.1 Fixed-dose combinations of BP-lowering drugs are highly encouraged by clinical guidelines.1 An innovative study, the QUARTET trial, tested the effect of a quadpill (containing irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg and bisoprolol 2.5 mg) or an indistinguishable monotherapy control (irbesartan 150 mg).11 The primary outcome was the difference in unattended office systolic BP at 12 weeks. Secondary outcomes included BP control (standard office BP <140/90 mmHg), safety and tolerability. By 12 weeks, 44 of 300 participants (15%) had additional BP medications in the intervention group compared with 115 of 291 participants (40%) in the control group. Systolic BP was lower by 6.9 mmHg (95% CI [4.9–8.9]; p<0.0001) and BP control rates were higher in the intervention group versus the control group: 76% versus 58% (RR 1.30; 95% CI [1.15–1.47]; p<0.0001). There was no difference in adverse-event-related treatment withdrawals at 12 weeks (p=0.27). Uptitration of BP treatments occurred more frequently among control participants than intervention participants (p<0.0001); nonetheless, at 52 weeks mean unattended systolic BP remained 7.7 mmHg lower and BP control rates higher in the intervention group (81% versus 62%; RR 1.32; 95% CI [1.16–1.50]). A subgroup continued randomly-assigned allocation to 12 months to assess long-term effects and the results were sustained.
Polypills
Cardiovascular polypills have also proven to increase adherence and BP control but – in contrast to BP fixed-dose combinations – also include lipid-lowering and antiplatelet drugs.12 TIPS-3 assessed the administration of polypill (simvastatin 40 mg, atenolol 100 mg, hydrochlorothiazide 25 mg and ramipril 10 mg) or placebo daily, aspirin (75 mg) or placebo daily, and vitamin D or placebo monthly.13 The study used a 2 × 2 × 2 factorial design and included 5,713 patients without cardiovascular disease but high cardiovascular risk. After 4.6 years of follow-up, subjects treated with the polypill plus aspirin had 31% lower rate (HR 0.69; 95% CI [0.50–0.97]) of the primary endpoint (death from cardiovascular causes, MI or stroke) compared to the combined-treatment group. The Polypill Trialists’ Collaboration performed a patient-level meta-analysis of 18,162 patients treated with polypills or standard treatments and results clearly supported the effect of the different polypills on BP control as well as significant reductions in MI, stroke and cardiovascular mortality incidence.14 A recent retrospective and propensity-score-matched study performed in Spain demonstrated that the use of the Fuster-CNIC polypill (aspirin 100 mg, atorvastatin 20–40 mg and ramipril 2.5–10 mg) was associated with higher rates of lipid and BP control and, moreover, lower rates of major cardiovascular events.15 The SECURE trial (Secondary Prevention of Cardiovascular Disease in the Elderly), an open-label randomised clinical trial comprising patients ≥65 years with recent acute coronary syndrome treated with the Fuster-CNIC polypill versus standard care, demonstrated a 24% reduction of major cardiovascular events in patients treated with a polypill, that was mainly driven by a 33% reduction in cardiovascular mortality.16 These results clearly support the benefit of polypills in terms of prognosis beyond risk factors control.
In conclusion, new formulations of recognised medications can increase BP control and reduce the burden of cardiovascular events.
Renal Denervation
The results of the SPYRAL Pivotal trial, combining evidence from the pilot and pivotal trials using a Bayesian design, were published in 2020.17 The trial was powered for change in mean 24-hour and office systolic BP between baseline and 3 months. A total of 331 patients were randomised either to renal denervation (n=166) or sham treatment (n=165). While mean 24-hour systolic BP did not change significantly in the sham group (−0.6 mmHg; 95% CI [−2.1, 0.9]), there was a significant reduction in the renal denervation group (−4.7 mmHg; 95% CI [−6.4, −2.9]). The primary endpoint, baseline-adjusted change in mean 24-hour systolic BP after 3 months, was reached. The between-group change in mean systolic BP was −3.9 mmHg (Bayesian 95% credible interval −6.2, −1.6). These positive results suggest the need to maintain research into this technique and to optimise patient selection.
Further study results are expected in the coming years, including the findings of three studies focusing on ultrasound-based renal denervation (RADIANCE HTN-TRIO [NCT02649426], REQUIRE [NCT02918305] and RADIANCE II [NCT03614260]. Chemical-mediated radial denervation using alcohol injection in the perivascular space of the renal arteries through microneedles is under investigation in the Target BP I (NCT02910414) and TARGET BP OFF-MED (NCT03503773) studies.18
New Approaches
Telemedicine
New strategies for diagnosis and care delivery are being studied. An innovative programme has been implemented in the Mass General Brigham health system with a remote, algorithmically driven disease-management programme that uses navigators and pharmacists, supported by specialists, to initiate and titrate medications.19 The analysis of 1,437 patients reported that 556 (39%) completed initial titration, 431 (30%) remained under active titration and 450 (31%) were referred to their primary care physician or expert clinics, withdrew, or became unreachable. Mean home systolic/diastolic BP reduction compared to programme entry was 14/6 mmHg (p<0.001 for both). No serious adverse programme-related outcomes occurred. The mean (± SD) number of calls and titrations per patient were 15 ± 9 and 2 ± 1 for the lipid and 27 ± 10 and 2 ± 1 for the hypertension programmes, respectively. This study demonstrates the efficacy and effectiveness of remotely delivered, navigator- and pharmacist-led, standardised algorithmic care of at-risk but undertreated patients to optimise guideline-directed therapy for lipids and hypertension across large populations.19
Another approach in this field is the use of personal devices. A recent report randomised 333 participants to a smartphone coaching app to promote home monitoring and hypertension-related behaviours on systolic BP level or a BP tracking app. The 6-month follow-up results did not find differences in home systolic BP, although patients in the smartphone app group reported higher self-confidence in controlling BP.20
Population Approaches
New strategies for the control of hypertension in large populations, such as simple, algorithmic, accessible, non-toxic and effective (SAANE) algorithms for hypertension to enable less skilled health workers working in more decentralised or remote clinics to successfully initiate, titrate, and maintain hypertension treatment with little supervision by more skilled health workers are being implemented. SAANE algorithms indicate clear titration steps but do not go as far as selecting a preferred algorithm or naming the preferred drug within a class.21 This strategy facilitates successful management of hypertension treatment by less skilled health workers with little supervision. Similarly, SAANE algorithms facilitate not only the initiation of therapy, but also adjustment to reach hypertension control.
Artificial Intelligence
Artificial intelligence is advantageous for hypertension management and can be used to establish clinical evidence for the practical management of hypertension.22 The advantages of artificial intelligence for the management of cardiovascular disease are numerous, but mostly rely on the capacity for studying large populations and providing differentiated patterns of disease and more personalised medicine.23
Specific Clinical Settings
Primary Prevention
Ground-breaking evidence in the field of primary prevention was provided by the HOPE-3 trials.24,25 HOPE-3 tested two BP lowering agents and a statin using a factorial design. The analysis on BP was performed in 12,705 participants at intermediate risk who did not have cardiovascular disease and were randomised to receive either candesartan 16 mg/day plus hydrochlorothiazide 12.5 mg/day or placebo.24 At baseline, the mean BP in the entire trial population was 138.1/81.9 mmHg and the average difference between both treatment arms during the follow-up was 6.0 ± 13.0 mmHg. No differences were observed in the two prespecified coprimary efficacy endpoints (composite of death from cardiovascular causes, nonfatal MI, or nonfatal stroke and the composite of these events plus resuscitated cardiac arrest, heart failure or revascularisation). Nonetheless, significant reductions were observed in patients with baseline systolic BP >143.5 mmHg for both efficacy endpoints. The results of the HOPE-3 support initiation of BP lowering therapies, even in combination, in patients with grade I hypertension.
Administration of rosuvastatin 10 mg/day provided a 24% reduction in major cardiovascular events (HR 0.76 95% CI [0.64–0.91]; p=0.002).25 These findings are in concordance with ASCOT, which demonstrated the benefit of the addition of atorvastatin to a BP-lowering drug in patients with high BP, and endorse the over-all cardiovascular risk assessment and treatment in patients with intermediate cardiovascular risk.26
Pregnancy
Hypertension and BP management during pregnancy remains a clinical challenge as well as a difficult scenario regarding clinical trials. Nonetheless, the CHAP trial tested the safety and efficacy of BP control to 140/90 mmHg in 2,408 pregnant women with mild chronic hypertension and singleton foetuses at a gestational age of <23 weeks. The strategy of targeting a BP of <140/90 mmHg reduced the incidence of a primary outcome (preeclampsia with severe features, medically indicated preterm birth at <35 weeks’ gestation, placental abruption or foetal or neonatal death), which was lower in the active-treatment group than in the control group (30.2% versus 37.0%), with an adjusted risk ratio of 0.82 (95% CI [0.74–0.92]; p<0.001). No differences were observed in the incidence of small-for-gestional-age birth weight (<10th percentile) in both groups: 11.2% in the active-treatment group and 10.4% in the control group (adjusted risk ratio 1.04; 95% CI [0.82–1.31]; p=0.76). The incidence of serious maternal complications was 2.1% and 2.8%, respectively (risk ratio 0.75; 95% CI [0.45–1.26]), and the incidence of severe neonatal complications was 2.0% and 2.6% (risk ratio 0.77; 95% CI [0.45–1.30]). The incidence of any preeclampsia in the two groups was 24.4% and 31.1%, respectively (risk ratio 0.79; 95% CI [0.69–0.89]) and the incidence of preterm birth was 27.5% and 31.4% (risk ratio 0.87; 95% CI [0.77–0.99]).27
Elderly
The unblinded, non-inferiority OPTIMISE trial examined whether antihypertensive medication reduction is feasible, safe, and not associated with loss of systolic BP control.28 A total of 569 patients ≥80 years with systolic BP <150 mmHg taking at least two different antihypertensive agents were randomised to a strategy of medication reduction (removal of one hypertension drug, n=282) or usual care (no changes in medication, n=287). The primary endpoint was maintaining systolic BP <150 mmHg at 12-week follow-up; 86% of the participants in the medication reduction group had a systolic BP <150 mmHg versus 88% in the usual care group, indicating no difference between the groups (adjusted risk reduction 0.98; p=0.01 for non-inferiority). As expected, systolic BP and diastolic BP increased significantly by 3.4 mmHg (95% CI [1.0–5.8]; p=0.005) and 2.2 mmHg (95% CI [0.9–3.6]; p=0.001) in the medication reduction group, respectively. Of the seven prespecified secondary endpoints, five showed no significant difference between the strategies. However, medication reduction was only sustainable in 187 (66.3%) participants at 12 weeks.
Young Patients
The prevalence of hypertension increases exponentially with age. Nonetheless, younger patients with high BP represent an interesting group as they are exposed to the long-term effects of elevated BP. This is reflected in current guideline recommendations of more intensive treatment targets for young patients without established cardiovascular disease.1 Current evidence and recommendations support hypertension screening and early diagnosis in subjects at high risk of hypertension and intensive lifestyle modifications (including diet, weight control and exercise) and medical treatment initiation if BP is >140/90 mmHg.1,29
Conclusion
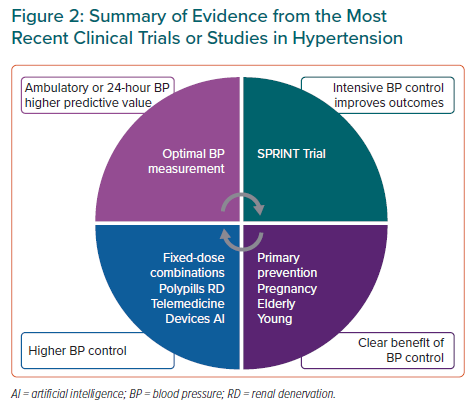
A summary of the most relevant clinical trial and studies is presented in Figure 2. Out-of-office BP measurement might be the most accurate method for BP monitoring and for the estimation of major cardiovascular events. Intensive BP control to a target systolic BP <120 mmHg in selected patients improves cardiovascular prognosis. BP control is also clearly beneficial in the pregnancy or the elderly. Different deliveries of care, such as fixed-dose combinations, polypills or renal denervation, improve BP control and this might be related to a lower incidence of cardiovascular events.1