Aortic stenosis (AS) is the most common valvular heart disease in Western countries, with a prevalence that increases with age.1–3 Aortic valve calcification (AVC) and fibrosis are the main pathophysiological changes that lead to degenerative AS. However, the relative contribution that each pathophysiological mechanism makes to the development of severe AS differs between men and women. For a similar degree of AS severity, men tend to have higher AVC than women.4–6 In contrast, women show more valvular fibrosis compared to men.7 As well as sex differences in the valvular lesion, sex differences in left ventricular (LV) remodelling and myocardial fibrosis in response to AS, have also been reported.8–10 Men demonstrate more LV systolic dysfunction, impaired LV global longitudinal strain, larger LV mass and volumes and more myocardial fibrosis compared with women. In addition, men show more concentric and eccentric LV hypertrophy, whereas women show more concentric LV remodelling.11,12 The interplay between the aortic valve lesion and the response of the LV to the pressure overload may differ between men and women and lead to differences in diagnosis rates of severe AS between both sexes. Although echocardiography is the imaging technique of first choice to diagnose severe AS, accurate characterisation of the phenotype of AS in terms of the anatomical lesion, haemodynamic severity and cardiac remodelling response to the pressure overload requires additional imaging techniques such as CT and cardiac magnetic resonance (CMR). These imaging techniques may be helpful in deciding the timing of interventions in patients who have severe AS but are asymptomatic and in patients in whom the diagnosis of the severity of AS is not clear with echocardiography and when symptoms cannot be explained by other causes (i.e. coronary artery disease and other comorbidities). Furthermore, the use of nuclear imaging techniques has facilitated an understanding of the degeneration process of the aortic valve, which may differ between men and women, and may help to develop medical treatments that will halt the AS degenerative process.
The imaging insights in AS recently reported are summarised in this viewpoint and their implications for clinical management of men and women with severe AS are appraised.
Imaging Sex Differences in the Pathophysiology of Aortic Stenosis
Degenerative AS is mainly caused by the calcification of a tricuspid aortic valve.13,14 Congenital abnormalities of the aortic valve have been described, the most frequent form being the bicuspid aortic valve, which may also degenerate over time. However, degenerative AS in patients with congenital abnormalities of the aortic valve presents at a younger age and more frequently in men compared with degenerative tricuspid AS.15,16
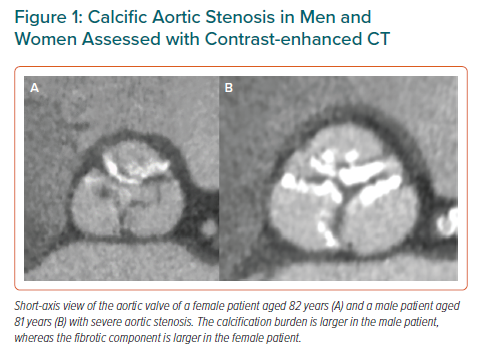
The underlying pathophysiological process of degenerative AS shares similar pathways to those of the coronary atherosclerosis process, but they are not completely identical. The initial changes consist of accumulation and oxidation of lipoproteins in the subendothelium, activation of inflammatory pathways, production of angiotensin-converting enzyme, up-regulation of adhesion molecules and matrix metalloproteinases and shear stress.17 Several studies have shown an association between degenerative AS and cardiovascular risk factors, such as diabetes, hypertension, hyperlipidaemia and smoking.18–20 A recent study in the general population showed a different interaction between sex, cardiovascular risk factors and the development of degenerative AS: while in men, dyslipidaemia was the main factor associated with AVC progression, in women, hypertension was the risk factor associated with AVC progression.21 Progression of the degenerative process of the aortic valve is followed by alterations in cellular and molecular pathways and in mineral metabolism that leads to leaflet thickening and stiffness. In this phase, oxidised lipoproteins stimulate valvular fibroblasts to release factors that lead to calcific nodule formation.22 There are also differences between men and women at this stage. For the same degree of severe AS, women show a lower AVC burden compared with men. Aggarwal et al. investigated the association between sex and AVC using ECG-gated non-contrast CT in 665 patients with AS.4 AVC load was quantified using the Agatston score method. The AVC load in women was lower compared with men (1,703 ± 1,321 arbitrary units [AU] versus 2,694 ± 1,628 AU, respectively). These results have been reproduced in other studies and suggest that women may show more fibrosis of the valve compared with men.6,7 Using contrast-enhanced CT, Cartlidge et al. showed the possibility of estimating the fibrocalcific ratio of the aortic valve representing the relative contribution of the valve fibrosis and calcification to the AS (Figure 1).23 The fibrocalcific ratio decreased with increasing AS severity and was higher in females than in males, indicating the more important contribution of fibrosis of the valve to AS grade compared with the calcific component in women. Consequently, sex-specific treatment pathways should be considered, as men show more calcification of the valve and women show more valvular fibrosis.
Besides non-contrast CT showing calcification of the aortic valve, PET combined with CT has been used to identify disease activity in the aortic valve. Two PET tracers,18F-fluorodeoxyglucose (18F-FDG) and 18F-sodium fluoride (18F-NaF) are known to target inflammation and calcification, respectively. The uptake of 18F-FDG, a glucose analogue, is increased in active metabolic cells such as vascular macrophages.2418F-NaF is a bone tracer used to detect calcium formation and remodelling.25 Marincheva-Savcheva et al. evaluated the 18F-FDG uptake in patients with AS compared with an age-matched control group.26 This study demonstrated that 18F-FDG uptake is increased in patients with AS. However, when subdividing the patients based on mild, moderate or severe AS, 18F-FDG uptake was increased in patients with mild and moderate AS, but not in those with severe AS, suggesting that inflammation is predominant during the early phase of degenerative AS. Dweck et al. investigated the uptake of 18F-FDG and 18F-NaF in the aortic valves of patients with normal aortic valves and those with various grades of AS.27 Patients with severe AS showed the highest uptake of 18F-NaF and 18F-FDG compared to those with normal aortic valves. Moreover, 18F-NaF uptake increased progressively with advancing disease severity, whereas 18F-FDG showed a more modest increase. However, the studies did not mention whether there were any sex differences in the uptake of 18F-FDG and 18F-NaF in patients with AS.
Imaging Sex Differences in the Phenotype of Aortic Stenosis: Diagnostic Challenges
Severe AS is diagnosed when the aortic valve shows restrictive motion of the cusps, the peak velocity of the aortic jet is >4 m/s, the mean transvalvular pressure gradient is >40 mmHg and the calculated aortic valve area (AVA) is <1 cm2 (or ≤0.6 cm2/m2 when indexed for body surface area). However, this diagnosis is not always straightforward because specific geometric assumptions and the influence of the loading conditions may lead to discordant grading. Low-flow (stroke volume index ≤35 ml/m2) low-gradient (≤40 mmHg) severe AS (AVA <1 cm2) can be observed in as much as 40% of patients, challenging the diagnosis of severe AS. Classical low-flow low-gradient severe AS is characterised by a left ventricular ejection fraction (LVEF) <50% and is more likely to be observed in men. In contrast, women more frequently show paradoxical low-flow low-gradient severe AS, characterised by a preserved LVEF.28
In patients with low-flow low-gradient severe AS, it is important to distinguish true-severe AS from non-severe AS (pseudo-severe AS). Low-dose dobutamine stress echocardiography (DSE) is the first recommended modality to confirm the diagnosis of true-severe AS in patients with low-flow low-gradient severe AS.29 True-severe AS can be considered if the AVA remains <1.0 cm2 and the mean gradient increases to ≥40 mmHg at any flow rate.30 Patients with classical low-flow low-gradient AS can fail to show an increase in stroke volume ≥20% during DSE. In these patients, the severity of AS cannot be accurately determined with DSE. The uncertainty of the diagnosis of severe AS also remains in patients with a significant increase in stroke volume but with small AVA and low gradient. In these patients, projected AVA at a normal flow rate is useful to grade the AS severity.31 A projected AVA <1.0 cm2 is considered as true-severe AS. This calculation is only feasible if there is an increase of ≥15% in mean transvalvular flow rate.
In patients with paradoxical low-flow low-gradient AS it is important to first identify whether there is an underlying cause of the low flow. If there is no underlying cause, then beside low-dose DSE, exercise-stress echocardiography can be used to determine the true severity of AS.32
However – as mentioned above – in some patients, the true severity of AS cannot be determined with DSE. In these patients, the severity of AS can be assessed with CT using the AVC score. In 646 patients with preserved LVEF and moderate and severe AS Clavel et al. showed different thresholds of AVC in men and women to define severe AS: 1,274 AU in women and 2,065 AU in men.33 These results were confirmed in a multicentre registry by Pawade et al. including 918 patients with at least mild AS and CT.34 The AVC threshold was 1,377 AU for women and 2,062 AU for men. These results led to the inclusion of AVC assessed with CT to refine the diagnosis of severe AS and using specific sex-specific cut-off values of AVC (Agatston score >1,200 AU in women and >2,000 AU in men).3
In addition, assessment of the acceleration time (AT) to ejection time (ET) ratio may help to assess the AS severity in these patients.35,36 Altes et al. evaluated the association between the AT/ET ratio and AS severity in 365 patients with low-gradient severe AS and preserved LVEF.36 An AT/ET ratio of >0.36 was an independent predictor of mortality in these patients. Einarsen et al. evaluated the association between increased AT/ET ratio and prognosis in 1,530 asymptomatic patients with mild-moderate AS and low-gradient severe AS.35 In the total study population, an AT/ET ratio ≥0.32 was significantly associated with a higher risk of cardiovascular death and heart failure hospitalisation. In patients with moderate AS, an AT/ET ratio of >0.37 was the optimal cut-off point to identify the patients at risk. However, it is important to note that the AT/ET ratio was not useful in the discrimination of women at risk of presenting all-cause death, cardiovascular death and heart failure hospitalisation. Therefore, we cannot conclude that this parameter will be useful in the decision making for women with low-gradient severe AS.
Imaging Sex Differences in the Aortic Root in Aortic Stenosis
Sex differences in the dimensions of the aortic root and ascending aorta are well-known. Accordingly, current guidelines recommend indexing the different segments of the aortic root and ascending aorta.37 Women have a smaller aortic valve annulus than men, which has an impact on the diagnosis of aortic stenosis severity.38,39 Smaller aortic valve annulus diameters lead to smaller AVA calculated by the continuity equation. In the SEAS trial population, indexing the AVA for body surface area increased the prevalence of severe AS from 31% to 44%.39 Interestingly, the increment in the prevalence of severe AS was more pronounced among men (from 23% to 42%; p<0.001) compared with women (from 43% to 46%; p=not significant). From the therapeutic point of view, a small aortic annulus has been associated with less favourable valve haemodynamics after aortic valve replacement (AVR), particularly when the AVR is performed surgically.38 Among 7,864 consecutive patients with severe AS undergoing AVR, 944 had an aortic valve annulus diameter ≤21 mm on transthoracic echocardiography, with 80% of them being female.38 After AVR, the mean transvalvular gradient and the rates of prosthesis–patient mismatch were significantly lower among those treated with transcatheter AVR compared with those treated with surgical AVR (12 ± 7 mmHg versus 15 ± 6 mmHg; p<0.001 and 14% versus 24%; p=0.001, respectively).
Furthermore, a smaller ascending aorta in women versus men has also diagnostic implications. A small ascending aorta is associated with a more pronounced pressure recovery phenomenon, not taken into consideration in the continuity equation. By correcting the AVA by the aortic area measured at the level of the sinotubular junction, the energy loss index is calculated. The energy loss index more accurately reflects the severity of AS.40,41 In the SEAS trial, patients with a small aortic root, defined by a diameter of the sinotubular junction indexed for body height of <1.4 cm/m in women and <1.5 cm/m in men, had more severe AS defined by AVA or indexed AVA but less severe when the energy loss index was used.42 A small aortic root was associated with the female sex and higher pressure recovery and lower LV mass index among other parameters. These studies were performed using echocardiography to assess the dimensions of the aortic root. However, it is well-known that CMR and CT have better spatial resolution to assess the dimensions of these structures.
Imaging Sex Differences in Left Ventricular Remodelling in Aortic Stenosis
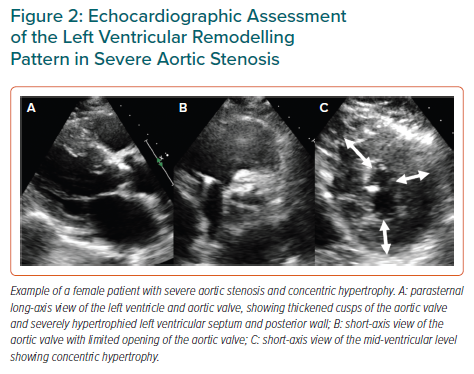
The LV pressure overload caused by AS leads to LV hypertrophy to maintain normal wall stress and preserved LV systolic function. However, excessive LV hypertrophy can eventually lead to myocardial oxygen supply–demand mismatch and myocardial fibrosis that can lead to LV dysfunction.43 Similar to the structural changes that occur in the aortic valve, the LV responds to the pressure overload differently in women and men. Echocardiographic studies have shown that men demonstrate more LV systolic dysfunction and larger LV mass and LV volumes while women more often show preserved LVEF, smaller LV mass and LV volumes.6,44,45 Based on the LV relative wall thickness and the LV mass, four different patterns of LV remodelling have been described. Among patients with AS, men more often show concentric and eccentric hypertrophy, whereas women more often show normal LV patterns of concentric remodelling (Figure 2).46 In particular, LV concentric hypertrophy and concentric remodelling have been associated with smaller stroke volume index and low-flow low-gradient severe AS. Lower stroke volume index (lower flow) has been associated with poor outcomes and sex-specific thresholds have been proposed: <40 ml/m2 for men and <32 ml/m2 for women.47 Furthermore, LV concentric hypertrophy is associated with a higher degree of myocyte apoptosis and replacement fibrosis, which is strongly associated with poor outcomes.48–50 The association between the type of LV remodelling and outcomes in women with severe AS and preserved LVEF differs from that observed in men: LV concentric hypertrophy was associated with 60% increased risk of all-cause or cardiovascular mortality in women whereas there was no significant association in men.46 These findings suggest that the structural changes that lead to LV concentric hypertrophy may be different for men and women and cannot be characterised with echocardiography alone.
CT
Contrast-enhanced CT can also be used for characterisation of LV remodelling in response to severe AS.10 This 3D imaging technique provides high spatial resolution images to assess LV mass and volumes. Furthermore, novel technologies allow the assessment of the extracellular volume as a measure of myocardial fibrosis.51 In contrast to echocardiographic studies, Kuneman et al. reported more LV concentric remodelling in men with severe AS compared with women, whereas the frequency of the other LV remodelling patterns was similar in both sexes.10 Furthermore, there was a significant association between LV concentric hypertrophy and poor survival in men, findings that contrast with the echocardiographic study.46 Differences in the type of patients and imaging techniques used to assess LV remodelling may explain the discrepant results of the studies.
LV myocardial extracellular volume can be quantified with CT using the data acquired without (baseline) and with contrast (3 minutes post-contrast pseudo equilibrium axial shuttle-mode scans).51 Using this technique, Scully et al. showed in 112 patients with severe AS (49% men) undergoing transcatheter aortic valve implantation that a high extracellular volume (>29.7%) was associated with poor survival after intervention.51 However, no details in terms of sex differences in extracellular volume were provided.
Cardiac Magnetic Resonance
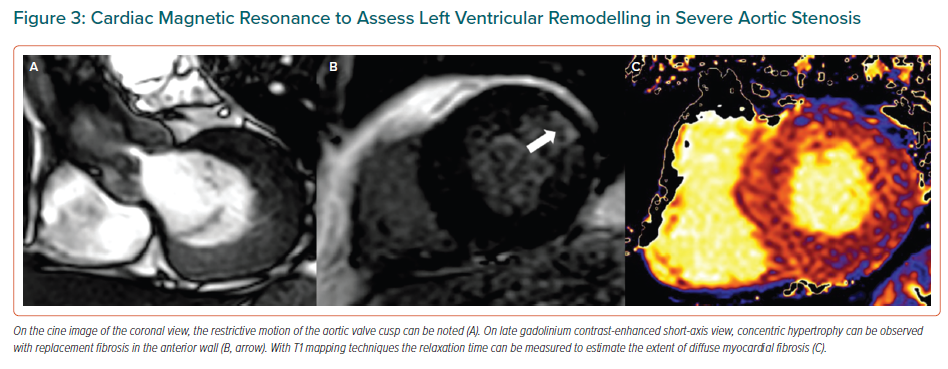
Besides being the reference standard for quantification of cardiac chamber dimensions and function, CMR is unique for providing characterisation of myocardial tissue. Using native and post-contrast T1 mapping sequences, several measures have been proposed to estimate diffuse myocardial fibrosis. In addition, late gadolinium contrast-enhanced (LGE) sequences provide information on myocardial replacement fibrosis (Figure 3). The amount of diffuse and replacement fibrosis has been associated with poor outcomes in patients with severe AS.48,52–54 Using CMR, differences in the response of the LV to pressure overload have also been reported between sexes. Studies including only patients with severe AS have demonstrated larger myocardial replacement fibrosis in men and similar extents of extracellular volume fraction between sexes.8,12 In the study by Singh et al., asymptomatic men with moderate and severe AS more frequently showed LV concentric remodelling and larger LGE and extracellular volume index compared with women.8 Interestingly, LV concentric remodelling was associated with new onset of symptoms only in men, suggesting that women with severe AS show a different pattern of remodelling that cannot reduce the wall stress and filling pressures in response to AS.8
In contrast, among 249 patients with at least mild AS, Tastet et al. showed that there were no statistically significant differences between men and women in terms of type of LV remodelling, although men showed a tendency to more often exhibit concentric hypertrophy.55 In addition, men showed larger LV mass than women but women presented with larger amounts of myocardial replacement fibrosis and extracellular volume fraction (diffuse fibrosis). Interestingly Treibel et al., showed that after AVR, focal myocardial replacement fibrosis is irreversible, but diffuse fibrosis regresses.56 These findings suggest that assessment of diffuse myocardial fibrosis may be used as a biomarker to determine the optimal timing of AVR and to monitor the response to AVR.
Clinical Implications
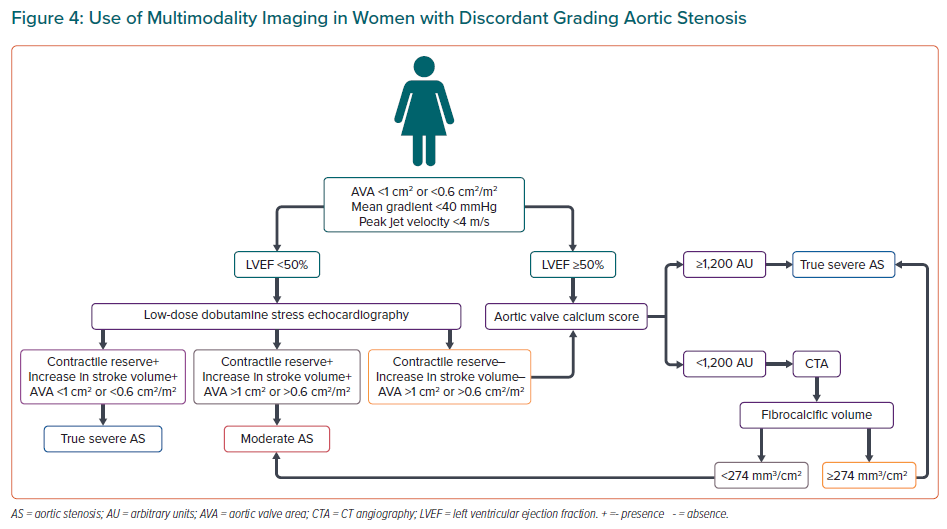
Challenges in the diagnosis of AS severity and the differences in the pathophysiology of AS (in terms of the degeneration process of the aortic valve and response of the LV to the pressure overload) lead to differences in the management and outcomes of men and women with severe AS. A recent large registry including 3,632 patients (42% women) with mild to moderate AS, showed that being female was associated with higher mortality and less referral to aortic valve intervention; this excess mortality was particularly observed among women with discordant grading AS.57 Acknowledging the differences in diagnosis, characterisation of the LV remodelling response and therapeutic management is the first step in performing prospective studies focused on the diagnostic yield of imaging tests and the efficacy of therapies (from medical therapies targeting the degeneration process of the valve to interventional therapies that aim at achieving the best valve haemodynamics and the best regression of LV remodelling) for men and women with AS. These studies will have an impact on current recommendation guidelines that acknowledge the lack of robust data on sex differences in management and outcomes in AS.3 Figure 4 proposes a diagnostic algorithm to facilitate the diagnosis of severe AS in women with discordant grading AS and to improve the referral to effective invasive treatments.