Aortic stenosis is the most frequent primary heart valve disease leading to surgery or catheter intervention in the Western world, with a growing prevalence due to the ageing population. No medical treatment can improve outcome above its natural history; the only treatments are surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI). The decision over type of intervention should take into account the patient’s cardiac and extra-cardiac features, their individual risk of surgery, which is assessed by the heart team using criteria including scores, the feasibility of TAVI and local experience.
According to current clinical practice guidelines in elderly patients at high surgical risk, TAVI is superior in terms of mortality to medical therapy in patients at extreme risk; non-inferior or superior to surgery in high-risk patients; and non-inferior to surgery and even superior when transfemoral access is feasible in intermediate-risk patients.1–7
The positive outcomes of TAVI have been demonstrated in several large-scale, nationwide registries, sustaining the generalisation of results in randomised controlled trials. This supports the use of TAVI over surgery in elderly patients at high surgical risk. Nevertheless, the choice between SAVR and TAVI (including the decision of access route) must be made by the heart team after careful individual evaluation.
Initial studies regarding subclinical leaflet thrombosis (SLT) began in 2015 and its natural history as well as its management and prognosis are still not well known. After cardiac CT first showed hypo-attenuated leaflet thickening (HALT) as the hallmark of SLT in a SAPIEN XT transcatheter aortic valve, Pache et al. evaluated the frequency of this phenomenon in 156 consecutive patients by dual-source CT angiography. They found that, irrespective of antiplatelet regimen, early HALT occurred in 10% of patients undergoing TAVI. Early HALT was clinically inapparent and reversible by full anticoagulation.8
Since this study, similar ones using cardiac CT (such as those of Makkar et al. and Chakravarty et al.) and even meta-analyses like the one by Rheude et al. have concluded in general that SLT occurs frequently in bioprosthetic aortic valves, and more commonly in transcatheter than in surgical valves.9–11
Although SLT is considered subclinical because it is fundamentally an imaging finding, there are discrepancies regarding its clinical relevance in the literature. There have been hypotheses suggesting that SLT may have clinical consequences, such as increasing the transvalvular gradient, being a precursor of thrombosis, reducing the durability of the prosthesis and increasing the likelihood of cerebrovascular events. However, these concerns are still a matter of debate and further studies are needed to clarify them.
On the other hand, from a therapeutic point of view, anticoagulation with new oral anticoagulants and warfarin, but not dual antiplatelet therapy (DAPT), has been shown to be effective in preventing and treating SLT. As a result, SLT is now recognised as a complication that follows TAVI. Its incidence varies, according to different studies, depending on whether cardiac CT is performed, so clearly it may be underdiagnosed. However, it is not a problem only for TAVI; it has also been described in surgical bioprostheses.9–11
Many gaps therefore still exist regarding this condition, despite growing interest and the numerous published studies. The time SLT appears after TAVI, its short- and long- term consequences and, of course, the best strategies for prevention and treatment are some examples of topics that are not completely known and need further analysis.9–11
In this article, we review aspects of subclinical and clinical thrombosis with special mention to the role of imaging tests.
Definition of Clinical and Subclinical Valve Thrombosis
Prosthetic valve thrombosis is a well-known consequence regarding mechanical valves and anticoagulation is required for life after implantation. However, it is seen much less observed in biological valves, including those implanted using TAVI, where patients receive antiplatelet therapy. Nevertheless, as previously mentioned, during recent years, studies have reported thrombosis in these patients and, furthermore, two conditions are now are well recognised: clinical valve thrombosis and SLT.12,13
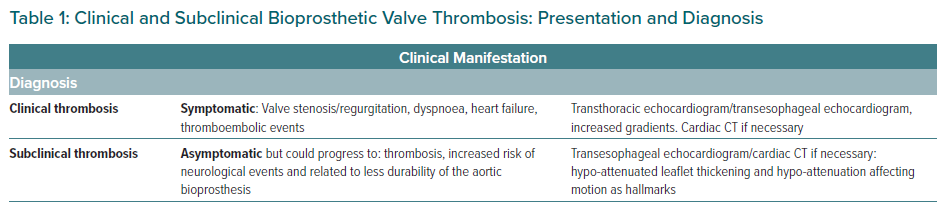
Clinical valvular thrombosis is identified as a clinical apparent prosthetic valve dysfunction with the characteristic finding of a mobile mass/thrombus in the prosthetic valve; it is diagnosed by either echocardiography or CT. It is due to a thrombus causing increased aortic gradients due to impaired leaflet coaptation or reduced leaflet motion. Differential diagnosis includes valvular degeneration, the most frequent being pannus or endocarditis. Symptoms, as described below, include heart failure or systemic embolic events.9,14,15
On the other hand, as Pache et al. first said using CT, SLT can be described as a hypo-attenuating defect at the aortic side of the leaflets, also called HALT, but with a normal transvalvular pressure gradient on echocardiography. If more than 50% of leaflet motion is affected, this phenomenon is defined as hypo-attenuation affecting motion (HAM). Because this is frequently an incidental finding with no clinically apparent valvular dysfunction, it is known as subclinical leaflet thrombosis.8,9
Magnitude: Incidence of Clinical and Subclinical Valve Thrombosis
After SAVR with a bioprosthesis, the incidence of clinical valve thrombosis has been reported at 0.3–6% depending on the series.12,16 This condition was later identified also after TAVI. Based on retrospective observational records, the incidence in these patients is estimated to range between 0.6% and 2.8%.17,18
However, the reported incidence of SLT depends on the strength of screening, the diagnostic criteria applied, and the imaging technique used. The incidence of this condition, both with and without reduced movement of leaflets, is in a wide range (7–35%) with regard to transcatheter valves.9,10 There are few studies reporting its incidence in surgical aortic bioprosthesis. In the SAVORY/RESOLVE registry mentioned above, the incidence in surgical valves was lower than in transcatheter valves. Of course, surgical patients were significantly younger and had fewer comorbidities.15,19 Finally, in a prospective study of an aortic sutureless bioprosthesis, a higher incidence was found, with up to 38% of patients having HALT and 28% showing HAM in CT.10
CT is now considered the standard method for diagnosis of SLT and its incidence depends mainly on the intensity of cardiac CT screening.
Pathophysiology and Predisposing Factors
Numerous physiopathological mechanisms have been proposed for the development of bioprosthetic valve thrombosis.
Rashid et al. referred to Virchow’s triad to explain the potential mechanisms underlying thrombosis. First is surface damage related to native aortic valves which are present in transcatheter aortic valve replacement: they are pushed aside in the sinus of Valsalva, which not only cause changes in valve geometry and haemodynamics but also can induce thrombosis due to exposure of tissue factor. Second are haemodynamic flow alterations and finally is a hypercoagulable state related to some host variables.20
Jaffer et al. reviewed the pathogenesis of clotting on blood contacting medical devices.21 Artificial surfaces promote clotting through complex processes including initial protein adsorption, which induces platelet adhesion, activation and aggregation; thrombin generation; and complement activation. Several factors have been associated with an increased risk of thrombus formation in the prosthetic surface, including mechanical factors during the implantation such as crimping and post-dilatation, and the nature of the tissue valves, with porcine valves having a higher risk than bovine ones.14,21,22
Furthermore, haemodynamic flow alterations across the prosthesis with turbulence at the leaflet surface level can contribute also to thrombus formation. Factors, such as underexpansion, malapposition, post-dilatation or native calcification, may also stimulate thrombosis. Intra-annular valves in which larger neo sinuses (between the prosthesis frame and the prosthesis leaflets) are created are also at increased risk of thrombosis due to the flow stagnation. Other scenarios such as low cardiac output with blood stasis also promotes hypercoagulability.20
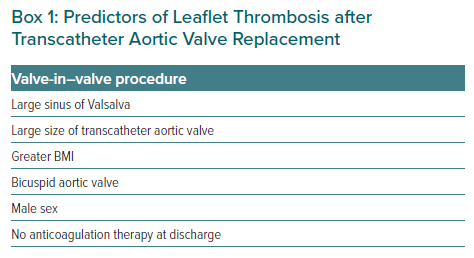
Moreover, some patient comorbidities and characteristics are associated with a pro-thrombotic and hypercoagulable state, such as an advanced age, diabetes, chronic kidney disease, heart failure, AF, chronic anaemia, cancer and smoking. All of them can both increase circulating thrombogenic factors and reduce their clearance.23 There are also procedural characteristics that predispose the patient to leaflet thrombosis after TAVI: valve-in-valve procedure, large diameter prosthesis, balloon-expandable prosthesis, underexpansion of the device, asymmetrical implantation and supra-annular implantation.24 Factors associated with an increased risk of thrombosis after TAVI are summarised in Box 1.14,20,25
Clinical Implications of Clinical and Subclinical Leaflet Thrombosis
The consequences of clinical valve thrombosis are secondary to valve stenosis or regurgitation and range from progressive dyspnoea to heart failure in a more acute form and depend on the degree of valve obstruction. Another possible manifestation is through a cardioembolic event such as a transient ischaemic attack, stroke or peripheral embolism. Thrombosis of a bioprosthetic valve is rare and is usually diagnosed in the early postoperative period.26
In subclinical leaflet thrombosis, patients are often asymptomatic but an increased risk of neurological events has been reported. It has been suggested that subclinical leaflet thrombosis has a possible negative effect on long-term valve durability and, of course, an increased risk of valve thrombosis and obstruction. For all of these, early detection should be essential.14,23 However, the suggested relationship between SLT in TAVI and neurological events is controversial at the moment because there is a discrepancy between the 10–15% prevalence of thrombosis in CT studies and 3–4% proportion of patients with stroke in large clinical trials.23,26 As Roseel et al. recently reported, although HAM was associated with an increased risk of transient ischaemic attack in the SAVOR/RESOLVE registry, this finding must be interpreted with caution because there was a longer time between the neurological event and the CT scan.14 At short- and mid-term follow-up, cerebrovascular events are similar following TAVI and SAVR.14,27
The hypothesis that subclinical thrombosis can affect long-term durability is a crucial issue; however, mid-term studies have demonstrated that TAVI durability is not inferior to surgical implantation of bioprosthetic valves.28
Finally, whether subclinical thrombosis is a substrate or precursor of valve thrombosis is not clear and needs further studies and follow-up.23,29
Table 1 summarises the main differences between clinical and subclinical valve thrombosis.
Diagnosis of Clinical and Subclinical Thrombosis
Echocardiographic Assessment after TAVI
As with for valvular heart disease and prosthesis, echocardiography is the main diagnostic tool for the assessment of TAVI.
The American and European societies of echocardiography have published a consensus document with recommendations for the use of echocardiography in TAVI. The Valve Academic Research Consortium has also elaborated a consensus document for systematic echocardiographic surveillance after TAVI. Furthermore, Pislaru et al. established the imaging approach for a proper assessment of TAVI to detect early and late complications.30 Transthoracic echocardiography (TTE) examination after implantation must be performed before discharge, at 6 and 12 months following implantation and every year thereafter.13,31,32
These consensus documents and recommendations established a detailed echocardiography assessment for prosthetic valve function. The first step is a visual inspection of the valve by 2D echocardiography and Doppler colour to evaluate the correct stent position and cusp mobility. It is essential to know the correct position of each prosthesis as both transcatheter aortic valve migration or malposition due to poor initial deployment or acute embolisation are early complications that must be prevented.
Next is haemodynamic analysis of traditional parameters by continuous wave Doppler: mean gradient; peak velocity; Doppler velocity index (DVI); and effective orifice area (EOA). Also, using Doppler colour, prosthetic and peri-prosthetic regurgitation have to be carefully evaluated.
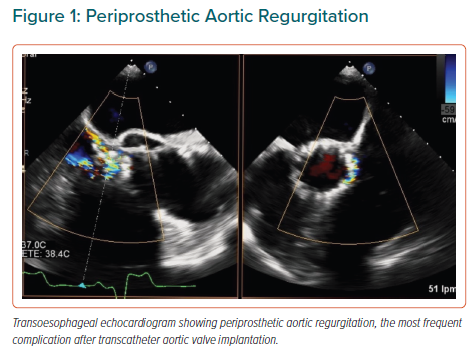
Complications such as TAVI thrombosis, obstruction, patient-prosthesis mismatch and regurgitation can be present and must be properly evaluated. Aortic regurgitation is, without any doubt, the most frequent and reported complication due to it causing excess of mortality and morbidity, and because measuring it is complex (Figure 1).
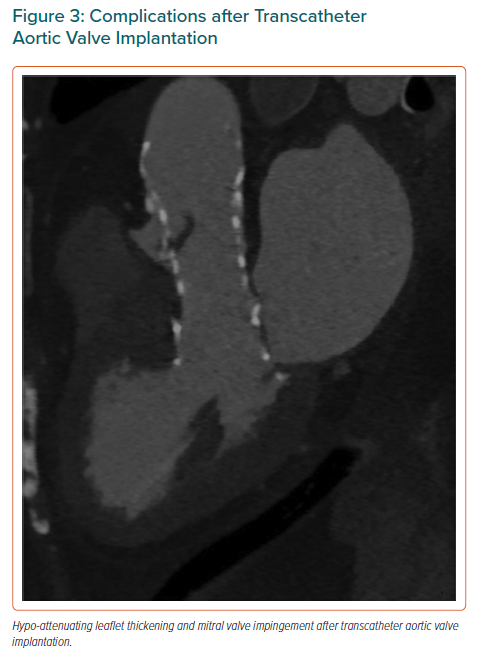
Other matters that have to be comprehensively analysed during echo assessment include mitral valve impingement, left and right ventricular size and function, and coronary artery obstruction.
During patient follow-up, comparative analysis with the basal study will be fundamental to determining valve function and complications. Suggested normal values for TAVI valves are gradient <20 mmHg, EOA 1.1 cm2 and DVI >0.35. Differential diagnosis of high gradients is similar to that for a surgical prosthesis.32
Clinical and Subclinical Thrombosis: from Transthoracic Echocardiogram to CT
Clinical thrombosis can have dramatic consequences and must be suspected by TTE if elevated transprosthetic gradients are present with reduce mobility of the cusps and increased leaflet thickness. However, TTE sensitivity for visualising thrombus formation is limited, and TEE and cardiac CT should be than the next diagnostic step.33
Cardiac CT is the gold standard diagnostic tool to assess the mobility and thickness of the leaflets. Jilaihawi et al. perfectly described the systematic methodology for the evaluation of leaflet thrombosis by CT.34 Their acquisition protocol used a contrast-enhanced, ECG-gated cardiac CT scan with full cardiac-cycle coverage with retrospective gating, which is necessary to assess leaflet motion. Retrospective imaging acquires continuous data through the cardiac cycle allowing the assessment of valvular motion in cine mode. Scan slice thickness should be submillimetre and heart rate controlled (below 70 BPM) to avoid artifacts is necessary. For most scanners, the pitch varies according to the patient’s heart rate, and the tube current–time product may vary with the patient’s body surface, whereas the voltage is usual near 120 kV. Contrast material is usually administrated as a bolus through an antecubital intravenous catheter, at a rate of 4–5 ml/s and the scan is initiated by bolus tracking on the ascending aorta. The volume of contrast material is variable; 60–80 ml can be used with the intention of achieving opacification of only the left-sided cardiac chambers and aorta.34,35
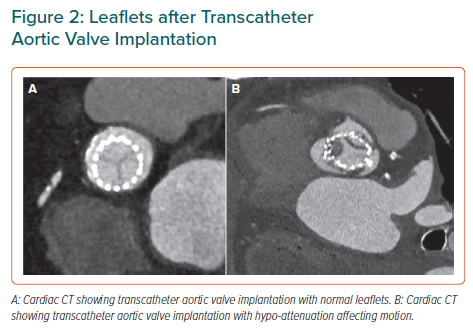
In subclinical leaflet thrombosis, patients are asymptomatic with transvalvular gradient measurements within the normal range. HALT and HAM are the CT diagnostic hallmarks. HALT (Supplementary Video) appears as a wedge shape or semilunar opacities of the leaflets that can be seen both in systole and diastole by 3D volume – rendered views. It can lead to reduced mobility of the leaflets (RELM), assessed in 4D volume-rendered CT. HAM is related to the presence of HALT and RELM at the same time with reduce leaflet excursion by more than 50% (Figures 2 and 3).35
The natural history of SLT is not known. The period during which it may start to develop is not restricted to a brief window after valve implantation but may develop over a prolonged period of time.
As Jose et al. describe, the development of obstructive TAVI thrombosis depends on the amount of thrombus, the number of leaflets involved and the duration of the phenomenon.36 They speculated it could be a progressive disease with an early phase where the predominant finding is just an imaging and subclinical abnormality and a late phase with an extensive thrombus, and a resultant gradients elevation of and manifestation of symptoms.36,37 HALT and HAM would be the earliest signs on imaging before clinical thrombosis with elevated transvalvular gradients.35,38 In addition, although SLT seems to be merely an imaging finding, NT-proBNP and D-dimer levels can be elevated. The relationship between SLT and clinical thrombosis is still controversial but, without a doubt, the detection of SLT clearly changes patient follow-up and treatment.14
From Prevention to Treatment: Gaps in Evidence
Uncertainty about optimal antithrombotic therapy after TAVI remains and the best treatment strategy for prevention and treatment of SLT is still not established.
Recently published American College of Cardiology and American Heart Association guidelines established that for patients with a bioprosthetic TAVI who are at low risk of bleeding, DAPT with aspirin 75–100 mg and clopidogrel 75 mg may be reasonable for 3–6 months after valve implantation, and that for patients with TAVI who are at low risk of bleeding, anticoagulation with a vitamin K antagonist (VKA) to achieve an INR of 2.5 may be reasonable for at least 3 months after valve implantation.39 European guidelines (published in 2017) recommend DAPT for 3–6 months followed by single antiplatelet therapy in all patients who do not require anticoagulation therapy.40
Otherwise, oral anticoagulant (OAC) therapy seems to prevent the development of both SLT and clinical thrombosis; at the same time, OAC restores leaflet motion in case of HAM.14 Sondergaard et al. suggested that patients on OAC were less likely to show progression than those on antiplatelet therapy or no antithrombotic therapy.37
In case of clinical valve thrombosis, treatment with VKA should be started and continued until valve function is restored.
Different antithrombotic strategies have been studied in several trials (the AUREA (NCT01642134), ATLANTIS (NCT02664649) and AVATAR [NCT02735902]), which will provide further evidence about the optimal antithrombotic treatment after TAVI. At the moment, there is no evidence to support OAC therapy for all patients after TAVI given the increased bleeding risk in this elderly group of patients.14
In the randomised ARTE trial, which compared aspirin plus clopidogrel with aspirin alone, patients in the single antiplatelet therapy arm experienced fewer major and life-threatening bleeding events without increasing risk of stroke.41 The GALILEO trial, which compare rivaroxaban plus aspirin with standard antiplatelet therapy was stopped prematurely due to higher risk of bleeding in the rivaroxaban group.14 Furthermore, GALILEO-4D, a substudy of the main GALILEO trial, aimed to evaluate the effect of a rivaroxaban-based strategy compared with an antiplatelet-based antithrombotic strategy on leaflet thickening and leaflet-motion abnormalities in patients with transcatheter aortic bioprostheses.
In patients with no indication for long-term anticoagulation after successful TAVI, a treatment strategy that included anticoagulation with rivaroxaban 10 mg once daily was more effective than an antiplatelet-based strategy in preventing subclinical reduced leaflet motion at 90 days. However, given the unfavourable clinical outcomes with rivaroxaban in the main GALILEO trial, the authors concluded that they could not recommend routine imaging for the detection of reduced leaflet motion or the routine use of anticoagulation after TAVI with the aim of preventing leaflet-motion abnormalities.42
A recent meta-analysis including the POPular TAVI trial, the ARTE trial, and the Dual Antiplatelet Therapy Versus Aspirin Alone in Patients Undergoing Transcatheter Aortic Valve Implantation trial concluded that, in patients without an indication for oral anticoagulation undergoing TAVI, aspirin alone significantly reduce the composite of thromboembolic and bleeding events, and do not increase the composite of thromboembolic events after transcatheter aortic valve implantation compared with DAPT.43 Questions about optimal strategies for prevention and treatment still exist and ongoing and future studies will probably help to solve them.
Conclusion
Many questions remain over SLT. HALT and HAM diagnosed by CT are the hallmarks of this condition, the incidence of which depends on the intensity of screening. Whether these phenomena are a surrogate for leaflet thrombosis reducing valve durability and increasing the risk of stroke is still controversial. More studies are needed to understand not only the natural history of HALT but also its clinical and prognostic implications and, what is more, whether screening and preventive strategies are needed. Further evidence is also needed about the most appropriate antithrombotic therapy after TAVI.