Hypertensive disorders of pregnancy (HDP) are a group of four disorders, whose definition depends on how far along in the pregnancy the hypertension occurs and the presence of maternal organ dysfunction: chronic hypertension (CH), gestational hypertension (GH), preeclampsia (PE) and superimposed preeclampsia (SPE). HDP is a complication seen in approximately 5–15% of all pregnancies.1–4 In previous years, evidence has shown that PE and its severe variant termed haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome are associated with a significant increased risk of maternal cardiovascular diseases (CVD) later in life including hypertension, coronary artery disease, heart failure, MI and stroke.5–11 PE/HELLP syndrome and CVD share common pathophysiological pathways and markers such as endothelial dysfunction, inflammation and angiogenic factors, which may explain this association.12 These findings have led to the acknowledgement of PE/HELLP syndrome as an important female-specific risk factor for CVD later in life by the American Heart Association (AHA), American Stroke Association and the European Society of Cardiology (ESC).13–15
As yet there is no consensus regarding clinical guidelines on how to optimally screen, prevent and manage CVD risk after pregnancies complicated in this way.16,17 The AHA recommends that healthcare professionals obtain pregnancy history in risk assessment for CVD, but this is infrequently implemented.13,17 Despite PE being listed as a risk enhancer of CVD by the AHA/ESC, women with a history of PE/HELLP syndrome and their doctors are often not aware of the increased risk. This may result in undetected hypertension and lack of adequate treatment and further cardiovascular risk assessment.18–20 Nonetheless, not all women who experienced PE/HELLP syndrome develop CVD later in life, indicating the existence of different levels of future risk. Currently, risk counselling for CVD is mainly based on traditional risk factors and biochemical markers (e.g. LDL and HDL cholesterol, triglycerides, glucose), only partially explaining the increased prevalence of CVD in these women.11,21,22 The identification of circulating non-traditional cardiovascular biomarkers of relevance for myocardial and coronary artery function may therefore be of additional value to determine which women are at greatest risk and to better understand the pathophysiological mechanism of CVD. Our understanding of these cardiovascular biomarkers in women has grown over the years. However, study designs vary widely and their findings have not been consistent. To date, a clear overview of the most promising biomarkers associated with CVD risk is lacking.23 The aim of this narrative review is to provide an update of the current literature on circulating non-traditional cardiovascular biomarkers in blood that may be associated with an increased cardiovascular risk in women after a previous PE/HELLP syndrome.
Review Methods
Classification and Definitions of Hypertensive Disorders of Pregnancy
PE and HELLP syndrome are characterised by hypertension developing after 20 weeks of gestation in combination with maternal organ dysfunction. In older definitions, proteinuria was mandatory, but this has been deleted in the latest guidelines.24,25 PE can be divided into early onset (e-PE) occurring before 34 weeks of gestation and late onset if it develops at 34 weeks of gestation or later (l-PE). The precise definitions of hypertension in pregnancy, PE, HELLP syndrome, GH, CH and SPE are presented in Supplementary Material Table 1.24–26
Source and Search Strategy
To find corresponding cardiovascular biomarkers, a literature search was performed in the PubMed database from inception to February 2021. The search was specified on cardiovascular biomarkers that may be associated with future CVD risk in women with a previous hypertensive disorder of pregnancy. Supplementary Material Table 2 presents the synonyms used to build a comprehensive search strategy. Further relevant studies were identified by examining the reference lists of the selected studies.
Study Selection
Observational studies, (systematic) reviews and meta-analyses were eligible for inclusion if written in English. First, titles and keywords were screened for containing terms for both PE/HELLP syndrome or HDP, as well as CVD or associated biomarkers. In addition, attention was paid to whether it concerns the risk of developing CVD in the future, not the risk of developing PE/HELLP syndrome. CVDs of interest were all pathological conditions involving the cardiovascular system including the heart, the blood vessels or the pericardium. In case there was doubt whether the study should be selected based on its title, the abstract was reviewed. Thereafter, the abstracts were screened for mentioning biomarkers in blood samples of women with (previous) PE/HELLP syndrome, linking this with CVD. Potential eligible studies were subsequently assessed by reading the full text, after which a final selection of publications was made. Studies comparing biomarker levels between women with a previous PE/HELLP syndrome and women who experienced uncomplicated, normotensive pregnancies were eligible for inclusion. Furthermore, studies investigating overlapping biomarkers between PE and any kind of CVD or studies evaluating the correlation between biomarkers and cardiovascular risk factors in women with (previous) PE/HELLP syndrome were eligible for inclusion. Biomarkers of interest were circulating non-traditional cardiovascular markers of relevance for myocardial and coronary artery function. Studies investigating only traditional markers were excluded (lipids, lipoproteins, markers of glucose metabolism, C-reactive protein). In order to gain information about women after a previous PE/HELLP syndrome, studies including only a case/group of women with GH, CH or SPE were excluded. Moreover, there was a preference for studies using a PE cohort or studies separately analysing the results of the different disorders of HDP in order to comment on PE/HELLP syndrome.
Outcomes of Interest
The primary outcome of interest is non-traditional cardiovascular biomarkers in blood that differentiate whether women with a history of PE/HELLP syndrome may have an elevated risk to develop any kind of CVD in the future or not. Another outcome measure of interest is cardiovascular biomarkers shown to be significantly different in women after previous PE/HELLP syndrome compared to controls or biomarkers correlated with cardiovascular risk factors in women with a history of PE/HELLP syndrome.
Results
Literature Identification
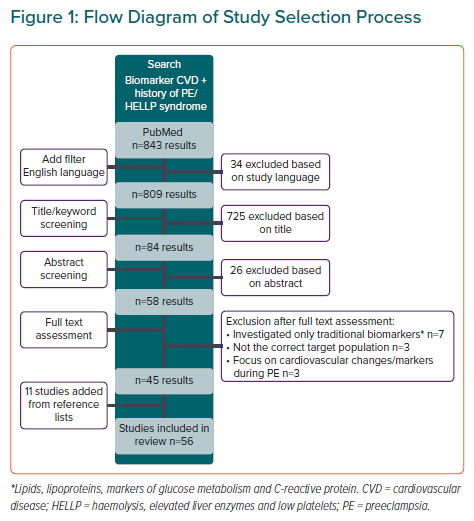
The process of study inclusion and exclusion is depicted in Figure 1. The initial search in PubMed yielded 843 articles. After removing non-English studies, 809 studies were screened for retrieval and finally 45 studies were included and 11 studies were added from the reference lists of selected studies. The total of 56 studies included 53 different non-traditional cardiovascular biomarkers.
Study Characteristics
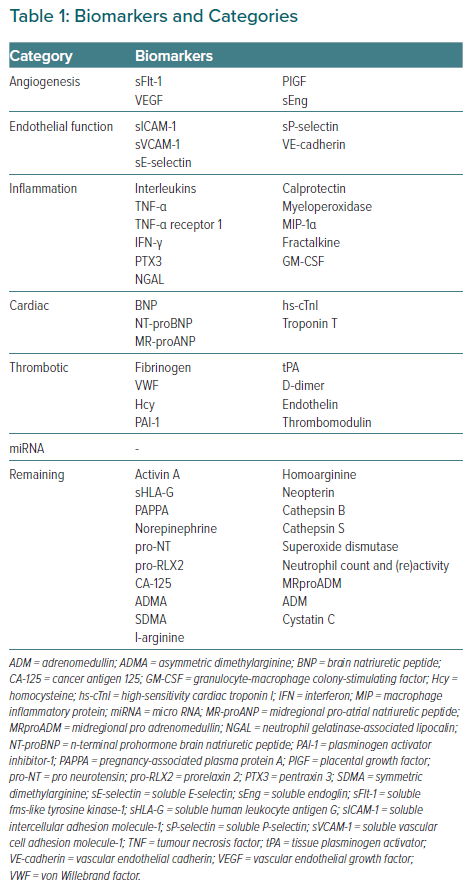
Study characteristics of included studies are shown in the Supplementary Material Table S3. Included studies were published from 2001 to 2021. Most studies assessed PE/HELLP syndrome separately from other HDP; four studies used a combined group of GH and PE or other pregnancy complications. As the definition of PE has changed over the years, there is a slight difference in study population between studies. The follow-up period varied from 3–6 days to decades after pregnancy. The 53 identified biomarkers were grouped according to their biochemical function (Table 1): angiogenesis, endothelial function, inflammation, cardiac, thrombotic, micro-RNA (miRNA) and a remaining group of biomarkers that do not fit well in one of these categories and/or have only been assessed in a single study.
For the following parts of the review, women with a history of PE/HELLP syndrome are referred to as cases and women with a history of uncomplicated normotensive pregnancy as controls. If another case and/or control-group is used in the included study, this will be described.
Biomarkers of Angiogenesis
Soluble fms-like Tyrosine Kinase-1 and Placental Growth Factor
Benschop et al. associated lower mid-pregnancy placental growth factor (PlGF) concentrations with worse cardiac structure and systolic blood pressure at 6–9 years postpartum in a mixed cohort of complicated and uncomplicated pregnancies. These associations persisted after exclusion of women with complicated pregnancies.27 Another study associated increased soluble fms-like tyrosine kinase-1 (sFlt-1) and decreased PlGF values in third trimester of PE with cardiovascular risk factors at 12 years postpartum, such as increased carotid intima media thickness (cIMT) measurements and worse lipid profiles.28 Neuman et al. found lower third trimester PlGF levels in women with PE with subsequent hypertension 1-year postpartum compared to women with PE without subsequent hypertension. However, third trimester PlGF and sFlt-1 values did not show significant value to predict hypertension at one year postpartum.29
In the first 10 years after delivery, several studies found some differences in biomarker levels between cases and controls. Kvehaugen et al. reported increased sFlt-1 values, but not PlGF, in cases both at delivery and 5–8 years postpartum.30 Three other studies reported significantly higher sFlt-1 levels in cases as well at 1, 1.5 and 4.5 years postpartum.31–33 Akhter et al. additionally observed a correlation between angiogenic factors and signs of arterial aging, measured by intima thickness and intima/media thickness ratio (I/M ratio).32 In contrast, Noori et al. and Yinon et al. found comparable sFlt-1 levels in cases and controls at 12 weeks and 6–24 months postpartum. However, they used smaller subject sample sizes compared to the above mentioned studies.34,35 Noori et al. did find higher PlGF levels 12 weeks postpartum in cases, whereas Escouto et al. found comparable levels in cases and controls at 6 weeks postpartum in a larger cohort of 288 cases.34,36
After a decade postpartum, four studies reported comparable sFlt-1 and PlGF levels in cases and controls.28,37–39 Finally, a meta-analysis found modestly higher sFlt-1 levels in women with previous HDP in a pooled analysis of 704 women (359 prior HDP and 345 controls). This difference was more pronounced when the sFlt-1 level was measured closer to the pregnancy (before 94 months postpartum).40
Soluble Endoglin and Vascular Endothelial Growth Factor
Four studies found comparable soluble endoglin (sEng) levels, and six studies mentioned comparable vascular endothelial growth factor (VEGF) levels in cases and controls in the postpartum period, ranging from weeks to 12 years postpartum.28,30,31,34,35,37,39,40
Biomarkers of Endothelial Function
Soluble Intercellular Adhesion Molecule-1 and Soluble Vascular Cell Adhesion Molecule-1
In the first decade after PE, seven studies reported no difference between cases and controls for soluble intercellular adhesion molecule-1 (sICAM-1) levels.23,33,41–45 Of note, one study even described lower levels 0.7 years postpartum in women with prior e-PE.46 Between 10–20 years postpartum, three studies described higher levels in cases and one study additionally related these high levels of sICAM-1 to features of the metabolic syndrome (MetS).38,47,48 In contrast, Tanz et al. reported comparable sICAM-1 levels 17 years postpartum in cases and controls.49 However, the use of a combined case group of PE and GH, and being based on women self-reporting makes the study less reliable.49 Studies including soluble vascular cell adhesion molecule-1 (sVCAM-1) levels, ranging from 1 to 20 years postpartum, have demonstrated no difference in cases and controls.23,28,33,38,39,43,45,48 Additionally, two meta-analyses found comparable sICAM-1 and sVCAM-1 levels in cases and controls.40,50
Soluble E-selectin
In a small study of 12 women with severe PE, there was a significant increase in soluble E-selectin (sE-selectin) in cases compared to controls at 3–6 days and then 12–15 weeks postpartum.51 Similarly, Chambers et al. reported higher levels in cases 3 years postpartum.42 Nonetheless, other studies have demonstrated no difference in sE-selectin at 1 and 2.5 years postpartum when comparing cases and controls.41,43 Four studies found comparable sE-selectin values 4.5–20 years postpartum in cases and controls, while Drost et al. described higher levels 10 years postpartum in a large cohort of 339 women with a history of e-PE.23,33,37,47,48
Soluble P-selectin, Soluble Intercellular Adhesion Molecule-3 and Vascular Endothelial-Cadherin
Soluble P-selectin, sICAM-3 and vascular endothelial-cadherin were mentioned in a single study, reporting no difference between cases and controls.37,51
Biomarkers of Inflammation
Interleukin 6 and Interleukin 10
Two studies reported higher interleukin (IL)-6 levels 12–14 weeks and 0.7 year postpartum in cases compared to controls.46,52 In contrast, three studies found comparable levels in cases and controls at 6 months, 1 and 2.5 years postpartum, with one of these studies examining a combined cohort of PE and GH.53–55 At 8, 9–16 and 17 years postpartum, three studies found higher levels of IL-6 in cases.47,49,56 One study observed a trend towards increased IL-6 levels and decreased IL-10 levels resulting in a higher IL-6/IL-10 ratio 20 years postpartum in cases compared to controls.57
Tumour Necrosis Factor α and Tumour Necrosis Factor Receptor 1
Vitoratos et al. found higher tumour necrosis factor (TNF)-α levels 12–14 weeks postpartum in a case-group of 17 women and Ehrenthal et al. showed higher values one year postpartum in a combined PE and GH group.52,54 However, six studies described comparable TNF-α levels in cases and controls, ranging from 6 months to 20 years postpartum, and one study even reported lower levels in cases 8 years postpartum.39,47,53,55–58 Östlund et al. found higher levels of TNF receptor 1 at 12 years postpartum in cases compared to controls.38
Other Markers of Inflammation
Two studies reported on interleukins other than IL-6 and IL-10, interferon-γ and macrophage inflammatory protein-1α at 6 months and 1.5–3.5 years postpartum. Both found comparable baseline levels but one study observed associations between inflammatory markers and multiple MetS parameters in cases and not controls and the other study showed alterations in the innate and adaptive immune response after a preeclamptic pregnancy.53,55 Finally, pentraxin-3, neutrophil gelatinase-associated lipocalin, granulocyte-macrophage colony-stimulating factor, fractalkine, calprotectin and myeloperoxidase showed no distinctive results.30,36,38,53,55,59
Biomarkers of Cardiac Function
Studies assessing postpartum levels of brain natriuretic peptide (BNP) and N-terminal prohormone BNP (NT-proBNP) described comparable levels in cases and controls.28,36,38,60 However, Alma et al. concluded that NT-proBNP and BNP differentiate both women with diastolic dysfunction from controls as well as women with preeclampsia from controls, implying common pathophysiology.61
Muijsers et al. found a strong association between high-sensitivity cardiac Troponin I (hs-cTnI) and blood pressure. Among formerly preeclamptic women, hs-cTnI levels were higher in currently hypertensive women compared to their normotensive counterparts.62
Levels of Troponin T and mid-regional pro atrial natriuretic peptide were comparable in cases and controls 12 and 5–8 years postpartum respectively.28,63
Biomarkers of Thrombosis
Fibrinogen and von Willebrand Factor
Six studies described comparable fibrinogen levels in cases and controls, ranging from 1 year to decade(s) after pregnancy.11,39,43,55,64,65 One study found increased fibrinogen levels 0.7 years postpartum in women with a previous e-PE compared to controls.46
Three studies found no difference in von Willebrand factor (VWF) values between cases and controls at 0.7-year, 1 year and decade(s) postpartum, whereas Blaauw et al. reported higher VWF levels 4.5 years postpartum in 17 women with prior e-PE.43,44,46,65 Furthermore, a meta-analysis showed no differences in cases and controls for these markers.50
Homocysteine
Five studies, of which three used a sample size of <20 cases, described comparable homocysteine (Hcy) levels in cases and controls at 3, 4.5, 5.5, 3-11 and 11 years postpartum.38,42,44,66,67 Three studies found higher levels in cases at 2.5, 8 years and decade(s) postpartum, although one study used a combined cohort of GH and PE, in which women self-reported making it less reliable.56,68,69 Wu et al. showed that cases with high Hcy levels have an increased arterial stiffness compared to cases with normal Hcy levels.70 Finally, a meta-analysis pooling 390 cases and 342 controls showed higher Hcy levels in cases.50
Plasminogen Activator Inhibitor-1, Tissue Plasminogen Activator, Endothelin-1, Thrombomodulin and D-dimer
Included studies described no significant difference in the postpartum levels of plasminogen activator inhibitor-1, tissue plasminogen activator, endothelin-1 and thrombomodulin in cases compared with controls.23,37,43,56,64,71 One study evaluated D-dimer levels 6 years postpartum and found higher levels in cases compared to controls.64
miRNA
Five studies were identified that assessed miRNA levels, all investigating different sets of miRNAs and therefore without confirmation in another study.72–76 Results of these studies are shown in Supplementary Material Table S4.
Other Biomarkers
Fifteen biomarkers examined to date show little potential: asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, homoarginine, neopterin, midregional pro adrenomedullin, adrenomedullin, cancer antigen 125, superoxide dismutase, cathepsin B, cathepsin S, cystatin C, pro neurotensin and prorelaxin 2 and neutrophil count and (re)activity.38,39,44,61,77,78 On the other hand, activin A, soluble human leukocyte antigen G (sHLA-G), pregnancy-associated plasma protein A (PAPPA) and norepinephrine did show interesting results. Shahul et al. showed that third trimester activin A levels in women with PE correlated positively with abnormal global longitudinal strain one year postpartum and that postpartum activin A level are associated with increased left ventricular mass index and worse diastolic function.79 Jacobsen et al. described that women with previous e-PE have higher levels of sHLA-G compared to controls at 1 and 3 years postpartum. This difference was not observed in the previous l-PE group.80 Drost et al. reported higher levels of PAPPA 10 years postpartum in women with a history of e-PE compared to controls after adjustment for traditional cardiovascular risk factors.23 Lampinen et al. found higher plasma norepinephrine levels 6 years postpartum in the resting lying supine position in cases compared to controls.71
Discussion
Main Findings
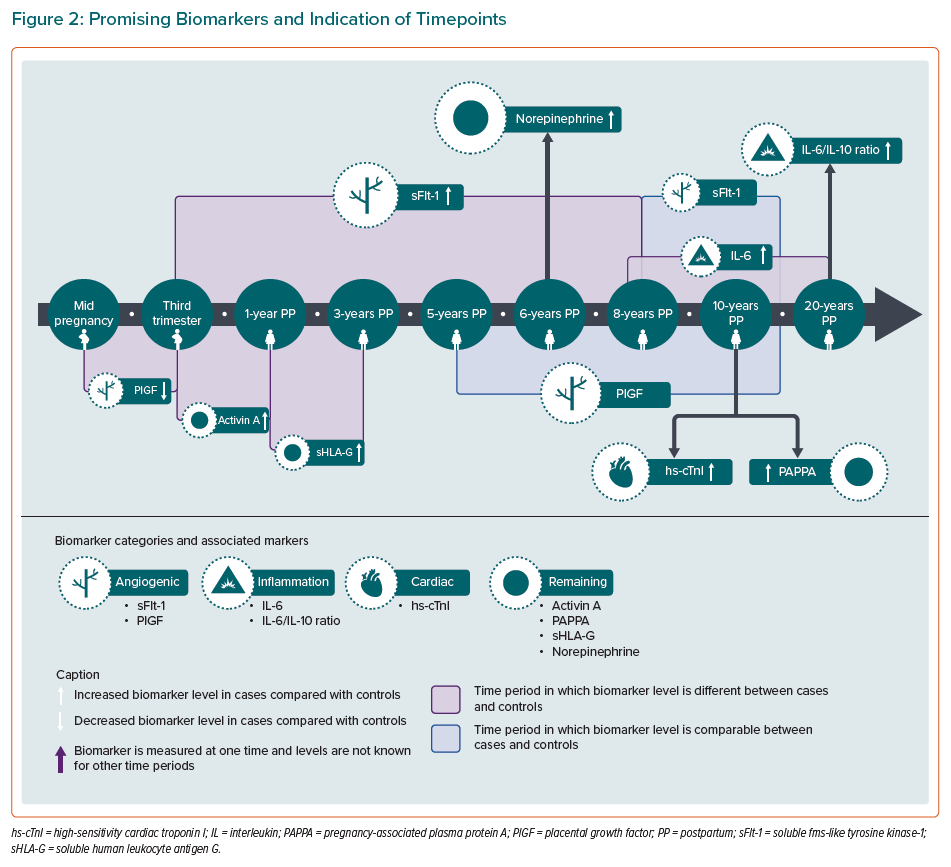
This narrative review presents an updated overview of the evidence available on non-traditional cardiovascular biomarkers in women with a previous PE/HELLP syndrome. After a complete review of the literature, nine biomarkers stand out as potential markers: sFlt-1, PlGF, IL-6, IL-6/IL-10 ratio, hs-cTnI, activin A, sHLA-G, PAPPA and norepinephrine.
The angiogenic marker sFlt-1 shows potential until 8 years after the index pregnancy as multiple studies show differences between cases and controls (not after 10 years postpartum). In addition, the association between the angiogenic markers sFlt-1 and PlGF and later CVD risk may be even stronger when these markers are determined during pregnancy (mid-pregnancy or third trimester). sFlt-1 is a soluble form of the VEGF receptor and neutralises both proangiogenic factors VEGF and PlGF by binding. An anti-angiogenic environment created by an increase in sFlt-1 (and sEng), and thereby decrease in PlGF and VEGF, plays a major role in the development of PE.81–84 Angiogenic factors are also associated with the development and progression of CVD. sFlt-1 levels are elevated in patients with heart failure and high levels are associated with adverse cardiovascular outcomes such as ischaemic heart disease.85–88 PlGF levels are also elevated in heart failure and are associated with intima thickening, pathophysiology of atherosclerosis, coronary heart disease and adverse cardiovascular events.89–93 Altered sFlt-1 and PlGF levels during pregnancy and the first few years postpartum may predispose these women for future CVD.
Markers of endothelial function examined to date show little potential. From the few studies that do find a difference and take time of biomarker determination into consideration, there seems to be some small trend towards increased sICAM-1 levels in cases more than 10 years postpartum, when compared with controls. However, the evidence remains too uncertain to conclude usability. In addition, results on sE-selectin are highly variable, making it unlikely to be meaningful for CVD risk stratification.
For the inflammatory biomarkers, IL-6 and the IL-6/IL-10 ratio appear to be the most promising. Years after PE, studies demonstrate a tendency towards increased proinflammatory IL-6 and decreased anti-inflammatory IL-10 levels, therefore the ratio could increase the sensitivity for detecting (subclinical) inflammation. Studies on other inflammatory biomarkers mainly showed comparable levels in cases and controls. However, two studies showed no baseline differences but alterations in inflammatory response and unique correlations between inflammatory markers and MetS in women with previous PE. Together this might hint that the alteration in baseline levels after PE is very small, or not measurable, and that an enhanced inflammatory response or unique associations explain the increased CVD risk. Inflammation plays a major role in the pathogenesis of PE, but also in atherosclerosis and coronary artery disease.94–100 Small changes in inflammatory baseline levels or response after a pregnancy with PE may explain the link with CVD.
The cardiac biomarker hs-cTnI appears to be promising as it is significantly higher in hypertensive women with a history of PE compared to their normotensive counterparts. Hs-cTnI is a widely used marker for cardiac tissue damage, such as MI, coronary heart disease, heart failure and is also associated with hypertension.101–106 Elevated levels in formerly preeclamptic women with hypertension may indicate some ongoing myocardial cell injury. BNP, NT-proBNP and MR-proANP show comparable postpartum levels in cases and controls, but these markers are increased during a preeclamptic pregnancy and are predictive for PE.61,63,107 Further research is suggested to investigate whether BNP, NT-proBNP and MR-proANP levels in pregnancy are associated with CVD risk later in life as these are known markers of heart failure.108,109
As for the thrombotic markers, only studies on Hcy show some diversity. However, no obvious trend is manifest and other thrombotic markers show comparable postpartum levels in cases and controls.
miRNA is a relatively new category of biomarker and a wide variety of miRNAs are associated with cardiovascular diseases. However, there are just a few studies linking PE and future CVD using miRNAs. These studies mainly investigate different miRNAs and lack the ability to conclude causality. At this point it is thought to be too early to make a statement on the utility of miRNAs in CVD risk stratification after PE/HELLP syndrome and studies express the need for further research.72–76
Four biomarkers were noteworthy in the remaining category: activin A, sHLA-G, PAPPA and norepinephrine. Activin A is a member of the transforming growth factor-ß family and associated with myocardial remodelling, cardiac fibrosis and involved in the pathogenesis of heart failure.110–112 sHLA-G has immunosuppressive effects and high levels indicate low-grade proinflammatory state and have been linked to negative cardiovascular health outcomes, such as decreased ejection fraction and heart failure.113–116 PAPPA is a metalloproteinase and increased levels are associated with atherosclerotic plaques, acute coronary syndrome and cardiovascular death.117–119 Finally, elevated norepinephrine levels increase arterial pressure and promote atherosclerosis and insulin resistance.120–122
The most interesting non-traditional cardiovascular biomarkers, including notification of the time period in which the biomarker level differs between cases and controls, are presented in the timeline of Figure 2. These biomarkers suggest alterations in different pathways which together might contribute to the increased cardiovascular risk seen in these women postpartum.
Strengths and Limitations
In this review we assessed differences in non-traditional cardiovascular biomarker levels between women with a previous PE/HELLP syndrome and women with a prior uncomplicated pregnancy, taking the timepoint of biomarker measurement into account. Among the included studies were also two meta-analyses. These contain pooled studies with different points in time at follow-up, ranging from weeks to decades. However, it cannot be ruled out that there might be an (optimal) point in time when the biomarker levels between cases and controls do differ from each other. This is where the present review provides new insights: by separately analysing the different studies while taking the timepoint of biomarker analyses into account. It should be noted that apart from biomarkers, there are studies investigating the potential role of imaging techniques (e.g. measuring cIMT, I/M ratio) and vascular functional testing (e.g. pulse wave velocity, augmentation index, flow mediated dilation) to screen these women postpartum.32,35,39,43,44 The predictive value of imaging and functional testing was out-of-scope for this review. The focus was on biomarkers as these are considered to be more favourable because they are easier, faster and cheaper to measure. However, the combination of most potential circulating biomarkers and functional tests could be of significance in the whole process of biomarker validation.
This review has some limitations. First, some articles may have been missed by the search strategy because they do not use the keyword ‘biomarker’, or a synonym, but the name of the biomarker itself. However, the reference lists of included studies have been scanned, so it is highly likely that these initially missed articles have nevertheless been included in the review. Overall, studies on cardiovascular biomarkers in women after PE/HELLP syndrome are quite inconsistent. This is probably due to a variety of reasons: heterogeneity of included patients (early, late, severe or mild PE, combined cohort of PE and GH), difference in the definition of PE (in older studies proteinuria was required), dissimilarities in recognition of confounders (e.g. known risk factors such as smoking and obesity) and the subsequent statistical models to adjust for it, and finally the difference in time interval between pregnancy and biomarker assessment. Besides there might be some technical variability between studies, i.e. different analysing methods, within- and between-person variation in biomarker levels and the biomarker’s stability during sample storage. The overall conclusion on the potential value of the biomarkers in this review should therefore be interpreted with some caution. Additionally, all studies were performed during or after pregnancy, making it unclear whether differences in biomarker levels were already present prepartum. Studies assessing pre-pregnancy levels do not (yet) exist, but would give information whether PE/HELLP syndrome is at least partly caused by pre-existing cardiovascular risk factors, thus potentially due to reverse causality. Furthermore, as we conducted a narrative review, the quality of the included studies has not been systematically been assessed by multiple reviewers and according to strict criteria. Of note, many studies used small sample sizes of dozens of women with a history of PE/HELLP syndrome. Also, studies compared biomarker levels at a certain time postpartum in women with a history of PE and women with a prior uncomplicated pregnancy, without discussion of the clinical relevance and implication of found statistically significant differences. For some biomarkers the (postpartum) reference range is not known, making it questionable if increased values actually are abnormal. Additionally, due to the retrospective designs of most included studies, they are able to measure association between biomarkers and CVD risk factors, but such designs are not strong enough to draw conclusions on causality. Finally, the review’s conclusion of the biomarkers in the remaining category is based on just a single published study on each particular biomarker.
Perspective
This narrative review summarises current evidence in literature on potential of non-traditional cardiovascular biomarkers in the postpartum period of PE/HELLP syndrome. At this time, sFlt-1, PlGF, IL-6, IL-6/IL-10, hs-cTnI, Activin A, sHLA-G, PAPPA and norepinephrine appear to be potential biomarkers that warrant further investigation in this population of women. Further investigations should focus on the prospective assessment of these biomarkers in women with preeclampsia and investigate whether these markers are able to distinguish women who subsequently develop hypertension and CVD, compared to those who don’t. Biomarker validation might benefit from combination with imaging or functional tests in such prospective studies to establish a link with clinical implication and predictive value for CVD in this particular population.
Click here to view Supplementary Material.