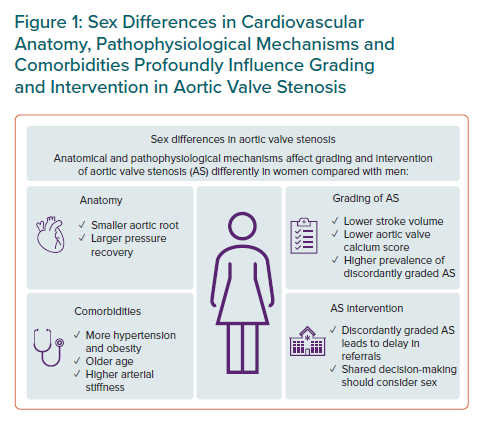
An increasing body of evidence demonstrates how sex influences the development of degenerative aortic valve stenosis (AS) at the aortic root, the valvular and the left ventricular (LV) levels. While the sex-specific impact of ageing and comorbidities promote differences in remodelling of the aortic root and the LV in AS, sex differences in tissue biology have been demonstrated in the aortic valve.1,2,3 These documented sex differences impact diagnosis, grading, treatment choice and outcome in AS. Still, sex-related differences are not well incorporated into current guidelines for the management of AS.4,5 In this special focus of European Cardiology Review, current evidence for sex differences in AS diagnosis and management is reviewed (Figure 1).6–8
Arterial hypertension is the most common comorbidity in AS patients and is significantly more common in older and elderly women than in men of similar age.9 Chronic arterial hypertension leads to stiffening of the arteries. Because of the increased stiffness, the backward pressure waves in the aorta travel faster and return to the aortic root during systole rather than in diastole. This increases the systolic blood pressure (BP) and decreases the diastolic BP in the aortic root, thereby reducing the drive of myocardial blood flow in the diastole and increasing the global LV load. In older subjects with arterial hypertension, women have higher arterial stiffness than men, contributing to the higher prevalence of uncontrolled systolic hypertension in women. It is well documented that a progressive reduction of transvalvular gradient is found in AS with increasing severity of arterial hypertension.10
Current European Association of Cardiovascular Imaging/European Association for Cardio-Thoracic Surgery and American College of Cardiology/American Heart Association guidelines both recommend re-assessment of AS when arterial hypertension is controlled.4,5 The European Society of Cardiology recently published a scientific document to guide better management of arterial hypertension in AS.11 In AS patients, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are recommended as first-line therapy, and in combination with β-blockers if insufficient BP control persists.11 However, BP control in elderly patients with established hypertension-associated organ damage such as arterial stiffness or LV hypertrophy is often not achievable, and is particularly difficult in women.12 Thus, concomitant arterial hypertension contributes to the higher prevalence of discordantly graded AS (a combined aortic valve area [AVA] ≤1.0 cm2 and mean transvalvular gradient <40 mmHg) found in women compared with men.
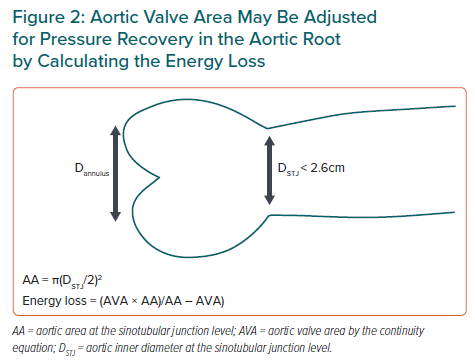
Chronic arterial hypertension also leads to remodelling of the arteries with increased wall thickness and reduced inner diameter. A small aortic root is therefore more common in women than in men independent of body size and is itself associated with a higher risk for cardiovascular (CV) morbidity and mortality even in patients with moderate AS.1 In patients with a small aortic root, larger pressure recovery occurs in AS, which influences the accuracy of AS grading by echocardiography using the continuity equation.13 In particular, the AS severity is overestimated in patients with a milder degree of AS.13 It is currently recommended to adjust for pressure recovery in the assessment of AS severity in patients with a small aortic root to avoid overestimation of the severity.6 This can easily be obtained by calculating energy loss (Figure 2).13
Obesity is nowadays a common comorbidity in AS patients, especially in women. Concomitant obesity has been associated with higher mortality in AS independent of sex. Obesity profoundly impacts the assessment of AVA and stroke volume when indexation for body surface area is used, contributing to the higher prevalence of both discordantly graded AS and low-flow severe AS in women.14 These subtypes of AS are less well understood, which may contribute to delays in referral and to suboptimal management of AS, particularly in elderly women. Sex differences in outcome were recently reported for patients with discordantly graded AS in an observational study from the Quebec Heart and Lung Institute.15 Typically, women with discordantly graded AS had a lower referral rate for valve replacement or intervention and had higher mortality than men with discordantly graded AS. Of note, women and men had the same prevalence of symptomatic discordantly graded AS in that study, but women were older, and had a higher prevalence of hypertension and a lower prevalence of coronary artery disease than their male counterparts.15
When discordantly graded AS is found based on AVA and mean gradient, current guidelines recommend assessment of stroke volume to identify low flow. By nature, women generally have both smaller aortic valve annulus size and LV volumes. Sex differences in LV remodelling during AS progression may potentiate these differences.9 These facts challenge the rationale for use of a non-sex-specific identification of low flow ≤35 ml/m2 in AS as recommended by current guidelines. Given that most AS patients today are overweight or obese, indexation of stroke volume and other key parameters to body surface area introduces further inaccuracy and should be avoided, in our opinion.
Although sex differences in LV remodelling in AS have been well documented in large studies using echocardiography, these differences may be further characterised by the use of multimodality imaging, including contrast-enhanced cardiac CT and cardiac MRI (CMR).8 Sex-specific thresholds for aortic valve calcium score by cardiac CT indicating the presence of severe AS have been well implemented in current guidelines to facilitate the recognition of severe AS in patients with low flow. Whether the additional assessment of new prognostic imaging parameters, such as fibrocalcific ratio in the aortic valve by contrast-enhanced CT or quantification of diffuse or replacement fibrosis in the myocardium by CMR, may improve the management of AS by better identification of optimal timing of valve intervention or replacement in women, remains to be demonstrated. In the meantime, it is time to use sex-specific threshold values for normal LV structure and function in AS, in concordance with guidelines for quantitative echocardiography, and to avoid indexation of stroke volume and other key parameters in AS evaluation to body surface area.
The invention of transcatheter aortic valve implantation (TAVI) has introduced a novel treatment option for elderly AS patients who are not candidates for surgical aortic valve replacement (SAVR).4,5 Although clinical and anatomical differences between women and men may influence the choice of TAVI versus SAVR, TAVI has also highlighted longevity and frailty as important aspects to be considered beyond what is technically feasible in the individual patient. It is important to consider sex- and age-related issues in a shared decision-making process.7 However, the basis for the best individual treatment decision remains an accurate and correct assessment of AS severity both in women and men.










