Patients with cardiovascular disease (CVD) often report non-restorative sleep. On the one hand this can be caused by the underlying disease (e.g. chronic heart failure) and on the other hand, sleep disorders are very common in this patient group. Sleep-disordered breathing (SDB), insomnia or a restless legs syndrome frequently lead to these symptoms.1,2 The importance of SDB in cardiology goes beyond symptoms of unrestful sleep, as it is an independent risk factor for the development and the progression of CVD.3 Due to the high prevalence of SDB in patients with CVD, the simple and non-invasive possibilities for diagnosing and treating the condition and its positive effects on CVD, cardiologists need to focus on the detection and careful evaluation of SDB.4
Sleep Apnoea
There are three basic mechanisms disrupting respiration during sleep:5
- obstruction of the upper airway;
- dysregulation of respiratory control; and
- hypoventilation.
Obstructive Sleep Apnoea
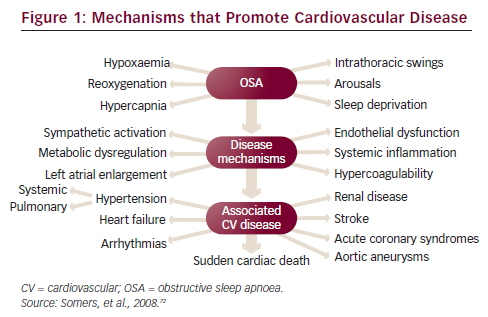
Obstructive sleep apnoea (OSA) is characterised by a recurrent partial (hypopnoea) or complete (apnoea) collapse of the upper airway during sleep, leading to hypoxemia and hypercapnia, large negative intrathoracic pressure swings, sympathetic activation, arousals and sleep fragmentation. The severity of the disease is determined by the number of respiratory events per hour of recording or sleep (apnoea–hypopnoea index [AHI]) and the number and severity of the oxygen desaturations. OSA is an independent risk factor for the development of CVD. There are several disease mechanisms that promote the development and the progression of CVD (see Figure 1).
Common risk factors for OSA are obesity, micrognathia, large tonsils, age and a post-menopausal state in women. Two-thirds of patients suffering from OSA are obese, while one-third has a normal body mass index. Typical symptoms of OSA are excessive daytime sleepiness, insomnia, morning headaches, depression, cognitive dysfunction, nocturnal dyspnoea, nocturia, erectile dysfunction and drowsy driving. Symptoms can vary, especially between male and female patients, and at least 50 % of patients with severe OSA do not report any symptoms of unrestful sleep. This number is even higher in patients with cardiovascular disease who very often do not report the typical symptoms, but rather symptoms of their underlying CVD.6
Standard therapy of OSA is continuous positive airway pressure (CPAP), which splints the upper airway and thereby avoids the collapse of the upper airway.7 Additional beneficial effects on the cardiovascular (CV) system, independent of the treatment of OSA, are an increase in intrathoracic pressure, a reduction of pre- and afterload and a reduction of the transmural left ventricular pressure, which can improve an impaired cardiac function.8–10 Long-term compliance with CPAP therapy in symptomatic patients is quite good with around 70 % still using the therapy regularly after five years.11
Central Sleep Apnoea and Cheyne-Stokes Respiration Central sleep apnoea (CSA) and Cheyne-Stokes breathing is mediated by a dysregulation of respiratory control. The most common identifiable cause for this is heart failure (HF), but it can also be seen in patients with stroke, especially acutely, and in renal failure. Cheyne-Stokes respiration (CSR) is characterised by periodic episodes of hyper- and hypoventilation, with a typical waxing and waning of the patient’s breathing. The dysregulation of respiratory control is caused by increased sensitivity of peripheral and central chemoreceptors. Other contributing factors are pulmonary congestion, a prolonged circulation time and often atrial fibrillation.12 CSR is not only a sleep-related breathing disorder, but can also occur during exercise and at rest in decompensated HF patients.13,14 CSA without a periodic breathing pattern can also be seen in HF patients.
As in OSA, CSA/CSR leads to desaturations and arousals activating the sympathetic nervous system. This can lead to a progression and deterioration of the underlying disease, and CSR has been shown to be an independent predictor of an increased risk for mortality in patients with HF and impaired left ventricular ejection fraction (LVEF).15,16 Basic therapy of CSA/CSR in CVD patients is the optimisation of the underlying disease, especially HF. In HF patients effective therapy with drugs and devices can significantly improve the severity of CSA/CSR.17–19 Heart transplantation cures CSA/CSR in HF patients.
Several therapies for CSA/CSR have been studied including oxygen, carbon dioxide, CPAP, bi-level positive airway pressure (BPAP) and adaptive servo-ventilation (ASV). Oxygen therapy has been studied in a few small-scale trials. Its use during sleep reduces the severity of CSA/CSR by approximately 50 %, but there is only one study showing clinical improvements.20 Administration of carbon dioxide can reduce the AHI but at the expense of hyperventilation and poor sleep quality, and is not used clinically.21
The early studies used CPAP as a treatment for CSA. Although not pacing breathing during sleep, CPAP improves CSA/CSR by increasing functional residual capacity and thereby oxygen stores, decreasing blood volume in the lungs and in the upper airway when lying down22 and by a direct effect on the paravasal juxtacapillary receptors (J-receptors) of the lung, reducing hyperventilation. In addition, CPAP reduces pre- and afterload and the transmural pressure.23
BPAP therapy can control CSA/CSR more effectively than CPAP24 but it can also worsen central SDB. ASV is the most effective treatment for CSA/CSR, delivering the best control of respiratory events during sleep.24 Dynamic pressure support during inspiration stabilises the respiratory pattern while controlling ventilation to avoid worsening of hyperventilation between the respiratory events. ASV also uses a so-called back up rate, which serves like a respiratory pacemaker during apnoeas. There are new treatment options currently being evaluated for the treatment of CSA/CSR. First results are available for phrenic nerve stimulation, which may improve central SDB by about 50 %.25
Hypertension
About one-third of patients with arterial hypertension suffer from clinically relevant OSA. In drug-resistant hypertension the prevalence is considerably higher, with up to 80 % of patients being affected.26 There is a causal relationship between OSA and the development of high blood pressure as has been shown in the Wisconsin Cohort Study.27 After four years, moderate to severe OSA increased the risk by three independent of other established risk factors. Additionally, there is a correlation between the severity of sleep apnoea and the loss of nocturnal dipping.28
CPAP reduces sympathetic drive in OSA patients and thus lowers blood pressure during sleep.29 The effect on daytime blood pressure is quite variable. Three meta-analyses on the effect of CPAP on blood pressure in OSA showed an average 24-hour reduction of 2 mmHg in the mean arterial pressure.30–32 The effects vary between no reduction to up to a 10 mmHg reduction.33,34 Different inclusion criteria, especially concerning the severity of sleep apnoea, the inclusion of normotensive patients and the presence of symptoms of daytime sleepiness are probably the reason for the large differences between the results. Sleepiness, severity of desaturations and compliance with CPAP therapy are important factors for a significant blood pressure reduction. Also pre-treatment with drugs plays an important role.35,36
The biggest effects of CPAP therapy for OSA can be seen in difficult-to-treat hypertension.37 Interestingly, a recent study showed improvements in sleep apnoea severity after renal sympathetic nerve ablation for resistant hypertension.38
Heart Failure
In a large cohort study39 (Sleep Heart Health Study) sleep apnoea was associated with a higher risk for developing HF. In patients with HF with reduced ejection fraction (EF) untreated SDB, OSA and CSA are independent risk factors for death.16,40,41 Also, the risk for malignant ventricular arrhythmias is increased.42
Up to 80 % of patients with chronic systolic HF suffer from SDB, with 45–50 % showing moderate to severe SDB.43 The biggest multicentre prevalence study (Sleep-Disordered Breathing in Heart Failure [SchlaHF] registry, NCT01500759) shows a prevalence of around 45 % for moderate to severe sleep apnoea in patients with symptomatic HF (New York Heart Association [NYHA] II and NYHA III) and a LVEF <45 %. It also shows that male gender, age, low EF, obesity and atrial fibrillation are clinical predictors of SDB in patients with chronic HF and that there is a significant gender difference, especially in patients under the age of 70. Much less attention has been paid to HF with preserved ejection fraction (HFPEF) where a similar prevalence of SDB can be found.44
Two randomised trials evaluating the effect of CPAP therapy for OSA in HF showed significant improvements in LVEF after one and three months.45,46 Additionally, improvements in quality of life and a reduction in sympathetic activity were shown. A Japanese cohort study demonstrated a positive effect of CPAP treatment for OSA in HF on survival while highlighting the importance of compliance.47 The existing data on OSA in CVD have led to a recommendation in the latest HF guidelines that OSA should be screened for and, if present, should be treated with CPAP in patients with heart failure.48 There are also data on OSA treatment for HFPEF showing improvements in the diastolic function of the left ventricle.49
While there is a general consensus on treating OSA with CPAP in HF, the discussion around the treatment for CSA/CSR is ongoing. A number of studies have shown that CPAP therapy improves cardiac function and symptoms in patients with CSA/CSR.23 This led, at that time, to the biggest sleep apnoea intervention study in the late 1990s. The Canadian Positive Airway Pressure (CANPAP) study investigated the effect of CPAP therapy of CSA/CSR in patients with stable HF on transplantation-free survival. While planning to enrol 406 patients, the study was terminated early for safety and futility after a pre-specified interim analysis, where 200 patients had been followed up for six months. Although CPAP attenuated CSA/CSR (AHI was decreased from 40 to 19/h), improved nocturnal oxygenation, reduced sympathetic activation and improved six-minute walking distance, neither the primary endpoint nor hospitalisations were different between the two groups.50 A post-hoc analysis of the CANPAP data showed a survival benefit for patients who had a better control of their respiratory events with CPAP, suggesting that effective control of SDB is important for improving morbidity and mortality in patients with CSA/CSR.51
ASV has been shown to be most effective in controlling SDB in CSA/CSR. There have been many small-scale trials showing improvements in sleep quality, quality of life, ejection fraction, natriuretic peptides and exercise capacity.52,53 Also, ASV is better tolerated compared with CPAP leading to better compliance.54 The effect of ASV treatment on morbidity and mortality is currently being investigated in the Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) study (NCT00733343), which aims to enrol approximately 1,260 patients with chronic stable HF and CSA/CSR. The results of the study are expected early 2015. It will also be interesting to see if the early positive results on the use of ASV for CSR/CSA in HFPEF will be supported by further results in this area.55
Arrhythmia
Severe OSA increases the risk of atrial fibrillation (AF), ventricular premature beats and non-sustained ventricular tachycardias (VTs) as well as the risk for sudden cardiac death during night-time.39,56 Bradycardia in the absence of structural cardiac alterations can be found in OSA and can be effectively treated with CPAP.57
Sleep apnoea is highly prevalent in patients with AF. More than 50 % of patients with paroxysmal and persistent AF show relevant SDB.58 OSA is an independent risk factor for developing AF.59 In a small study a significantly increased recurrence rate of AF after successful cardioversion was seen within 12 months in untreated OSA and also a significant risk reduction by treatment of OSA with CPAP.60
A meta-analysis on the recurrence risk of AF in OSA, even after catheter ablation, shows a risk ratio of 1.25 for the recurrence in patients who were diagnosed with OSA.61 This risk is even higher (1.40) if OSA is diagnosed with polysomnography, while it is not significantly increased (1.07) if only the risk for OSA is assessed with the Berlin questionnaire. If OSA is treated with CPAP, the recurrence risk can be reduced after ablation therapy.62
Coronary Artery Disease
Several studies have shown a high prevalence of OSA in patients with coronary artery disease (CAD).63,64 In patients with OSA without documented CAD nocturnal ST-segment changes can be found.65 Nocturnal ischaemic events are commonly seen in CAD patients with OSA and are associated with desaturations.66 Treatment of OSA with CPAP can alleviate nocturnal ischaemia.67 OSA may be a prognostic factor in patients with CAD and may increase the risk for a myocardial infarction.68,69 Two large cohort studies have demonstrated an increase in overall cardiovascular events in patients with untreated OSA.70,71 The risk of developing CAD is significantly increased in severe OSA (16.2 % versus 5.4 %, after seven years). An effective therapy of OSA can positively influence this risk.69
Conclusion
SDB is highly prevalent in patients with CVD. It increases the risk of developing CVD and worsens the existing disease, and there is increasing evidence that its treatment can improve mortality, morbidity and quality of life. Diagnosis and treatment of SDB should be considered in all patients presenting with CVD or CHF.