Permanent pacemakers (PPMs) have undergone rapid advancements in technology since their initial inception in the 1950s, with improvements to reduce generator size, increase battery life and quality, refine pacing algorithms and ensure lead fidelity. Despite these technological advances, the two points of weakness for the transvenous system remain the lead and the subcutaneous pocket, which can cause severe complications, including lead displacement, cardiac tamponade, pneumothorax, lead fracture and infection.
The leadless permanent pacemaker (LPPM) system was developed as a way to bypass these areas of weakness. Advances in battery technology and deep miniaturisation of electronics now offer the ability to implant the whole system into the right ventricle (RV).
LPPM constitutes an excellent alternative epicardial approach in case of specific comorbidities, such as limited upper venous access, recurrent or bilateral PPM infection and kidney failure with limited venous access.1 The complication rates with LPPM are expected to be lower through the avoidance of pocket-related and lead-related complications. The LEADLESS trial had an overall complication-free rate of 94%, and the Leadless II trial showed device-related serious adverse events in 6.7% of the patients.2,3 Real-world results of using a leadless pacemaker system in 1,817 patients reported serious adverse events in 2.7% of patients.4 The prevalence of leadless device infections is low as the principal sources of infection – the subdermal surgical pocket and pacemaker leads – are absent.
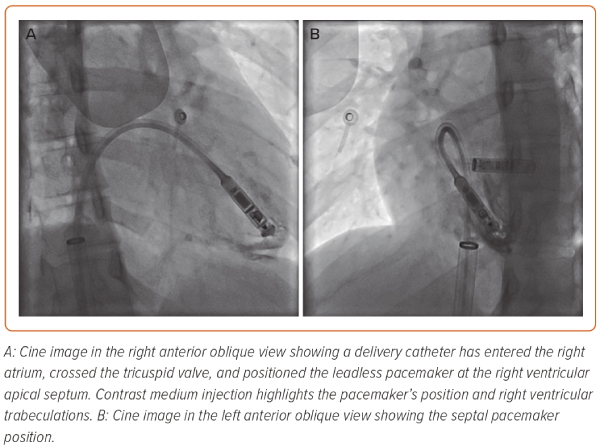
Devices that have been used clinically include the Nanostim (Abbott Medical) and the Micra (Medtronic). These devices are implanted percutaneously through femoral venous access and provide self-contained, intra-cardiac single-chamber RV pacing (Figure 1). Early trial data demonstrated the feasibility and safety of self-contained LPPM implantation, while pre-specified pacing and sensing requirements were also met in most device recipients.2,3
Unfortunately, Nanostim implantation was halted in October 2016 after incidences of battery malfunction were detected, resulting in sudden loss of pacing output and telemetry communication. There were no instances of patient injury, although device retrieval attempts were required and pacemaker replacement was recommended for pacing-dependent patients.5
Current Indications
At the beginning, the indication for LPPM therapy was mainly limited to patients who had persistent or permanent AF with slow ventricular response.2 However, as every additionally implanted pacemaker lead carries an incremental risk of complications, the indication area for RV VVI pacing (ventricle-only antibradycardia pacing) and LPPM is broad.6 Thus, LPPM may also be indicated in patients with paroxysmal atrioventricular (AV) block, sinus node disease or syncope in which no frequent ventricular pacing is expected.
The reduction in all-cause complications and infection observed with the Micra may be particularly attractive in patients with low pacing burden. Piccini et al. compared patients with and without persistent/permanent AF undergoing Micra implantation.7 Nearly one-third of patients selected to receive Micra were for indications not associated with AF. These patients required less frequent pacing compared with patients with persistent or permanent AF. In addition, this study showed that risks of syncope, congestive heart failure or pacemaker syndrome events were very low and did not differ in those with or without AF. LPPM can be a good alternative in patients with previous recurrent device infections or in individuals at high risk of systemic infection. Indeed, no recurrent device infections were reported in patients in whom an LPPM was implanted after extraction of an infected transvenous pacemaker.8
Some case reports mention implantation of LLPMs in patients with obstruction of the venous route, such as bilateral venous thoracic outlet syndrome and chronic obstruction of the superior vena cava.9,10
A European Heart Rhythm Association (EHRA) survey regarding the use of LPPM in Europe revealed that among 52 centres from 21 countries which participated in the survey the most commonly reported indications for LPPM were permanent AF (83%), a history of complications with a conventional pacemaker (87%), an anticipated difficult vascular access (91%) and an expected higher risk of infection (70%).11
More interestingly, LPPM is also considered in patients with otherwise standard indications for dual-chamber pacemakers when the anticipated pacing burden is expected to be very low (74%), in patients with very advanced age (61%) and in very active or non-cooperative patients who have a high risk of damaging the leads (39%).
The main reasons reported for not implanting LPPM included limited availability (36%), economic issues, such as lack of reimbursement (this accounted for 55% of centres not using LPPM), the high cost of the device (91% of centres), issues associated with patient selection, such as the lack of dual-chamber or cardiac resynchronisation therapy (CRT) pacing functions (27% of centres), or the absence of eligible patients (18% of centres). Lack of training was infrequently reported as the reason for not implanting LPPM (9%).
Indications in Specialised Patient Groups
Pace and Ablate Strategy
Three non-randomised studies have assessed the feasibility and safety of concurrent Micra leadless transcatheter pacemaker implantation and AV node ablation and showed no significant differences compared with a pace and ablate strategy using traditional transvenous pacemakers in both acute- and intermediate-term follow-up.12–14
Vasovagal Syncope
According to international guidelines and consensus, pacing should be considered only for patients over 40 years. A point of discussion is whether young adults with neurocardiogenic syncope of the cardioinhibitory type should be offered LPPM. Specifically, young patients who suffer from severe recurrent vasovagal syncope might be better served by backup single-chamber ventricular pacing.15,16
We believe that it is justified to offer LPPM as an alternative to conventional PPMs to young patients with a proven risk of cardioinhibitory syncope, given that they may have to live with the risks that PPM leads bring for decades. Moreover, there is a cosmetic advantage, without pectoral pocket formation, in men and women with neurocardiogenic syncope of the cardioinhibitory type.
Patients on Haemodialysis
Leadless pacing should be strongly considered in patients on haemodialysis to spare the upper venous system and access for dialysis. People on haemodialysis may also have an increased rate of transient bacteraemia while being dialysed, leading to haematogenous lead infection. So far, pacemaker infections have not been reported in leadless systems, presumably due to high blood flow velocities, small device size and encapsulation in the heart.
Congenital Heart Disease
LLPM is increasingly being considered for adults and children with congenital heart disease. The leadless strategy is potentially advantageous for patients with restricted superior venous access to the heart due to previous corrective surgery, intracardiac shunts or prior tricuspid valve surgery.17,18 Implantation in a morphological left ventricle (LV) has also been reported.19,20 However, long-term pacing characteristics in a highly diseased ventricle and the role of endothelisation of the LLPM in preventing thromboembolism in an arterialised circulation remain to be determined.
Device Retrieval
Both pioneering leadless devices were designed to be retrievable. The Nanostim pacemaker employs a specially designed catheter and single-loop snare system with an 18F sheath. The catheter is introduced via the femoral vein and used to position the snare to grasp a docking button on the device. After docking, a protective sleeve is advanced halfway over the device and rotated counterclockwise to unscrew it from the endocardium and cover it with the sleeve for retrieval.
Retrieval of the Micra leadless pacing system can be achieved using two different approaches. A 7 mm loop snare can be introduced through the Micra delivery system and once the Micra has been successfully captured with the snare, the retrieval cup is used for device recapture.21 This approach gives the ability to deliver countertraction with the Micra sheath. In the second approach, a steerable sheath is used in conjunction with a 20 mm snare. This provides the advantage of a larger snare size, with the downside of having no countertraction which could lead to potential myocardial damage.21
Effectively, the LPPM can either be extracted or abandoned before implanting a new device. In older devices, encapsulation may be an issue and abandonment could be a reasonable strategy. Unsuccessful retrieval is caused by complete encapsulation of the device, but the degree of Micra encapsulation is unpredictable. In the event of retrieval failure, the LPPM can be abandoned and a new system should be implanted at a different location in the RV, which is the recommended strategy from the manufacturer to reduce the risk of complications. However, abandonment may not be an appealing option, particularly in younger patients who can be expected to need several pacemaker replacements in their lifetime. There is no definitive answer as to how many devices can be implanted and abandoned before this becomes problematic or whether there are long-term risks of an abandoned LPPM. Surgical removal of the LPPM is an option when absolutely indicated and when percutaneous retrieval is not possible.
Incoming and Future Developments
Micra AV
Approved by the Food and Drug Administration in 2020, Micra AV extends the benefits of leadless pacing to more patients. Identical in size and shape to the original Micra, Micra AV uses an internal sensor within the device to sense movement in the RA. Sensing this movement allows the device to make calculated adjustments and pace the ventricle in coordination with the atrium, providing AV synchronous pacing therapy.
Micra AV performs ventricle-only pacing, dual-chamber sensing (VDD) with atrial tracking based on intracardiac accelerometer data. Four distinct segments of the cardiac cycle can be recognised: isovolumic contraction and mitral/tricuspid valve closure, aortic/pulmonary valve closure, passive ventricular filling and atrial contraction.22 Passive ventricular filling and atrial contraction were associated with mitral flow E and A-wave segments and were used to develop the algorithm to recognise atrial contraction and set timing cycles for VDD pacing.
The MARVEL 2 study showed feasibility and success of VDD programming of the Micra device with an improvement in AV synchrony from 26.8% during ventricle-only pacing and sensing to 89.2% during VDD pacing.23 No pauses or episodes of oversensing-induced tachycardia were reported during VDD pacing.
The advent of atrial tracking and effective VDD pacing for Micra could expand the patient indications to include those with normal sinus node function and AV block.
Leadless Dual-chamber Pacing
Another proposed advancement in LPPMs is dual-chamber pacing. In a prototype, this was achieved through two small leadless devices, one implanted in the atrium and one in the ventricle, which can communicate with each other efficiently and effectively to be able to achieve synchrony within each cardiac cycle. A novel technology for pacemaker communication uses the myocardium and blood as the transmission medium. This leadless dual-chamber pacemaker prototype was developed and successfully tested in vivo.24 Dual-chamber LPPM systems are expected to come to market to allow for atrial-based pacing.
Combining a Leadless Permanent Pacemaker Device with a Subcutaneous ICD
Existing leadless pacing systems and subcutaneous ICDs (S-ICDs) (Boston Scientific) are only available for patients requiring single-chamber RV pacing or shock-only defibrillation therapy, respectively.
In development is the EMPOWER Modular Pacing System, which includes a leadless pacemaker and the EMBLEM MRI S-ICD system and is designed to be backwards compatible with the EMBLEM S-ICD family. Whether patients with life-threatening arrhythmias subsequently develop a need for pacing or vice versa, this modular solution is designed to enable doctors to treat patients with the therapies they need.
The system will have the S-ICD send low-voltage 25 kHz signals from the shocking coil to the can that the leadless pacemaker will be able to sense. In the initial iteration, there is only one-way communication from the S-ICD to the leadless pacemaker.25
Anti-tachycardia pacing before or during S-ICD charging will be one of the main benefits of having the leadless pacemaker, as well as bradycardia pacing. Future iterations of the S-ICD system are also expected to allow bidirectional communication with other leadless devices in the modular cardiac rhythm management system.
Totally Leadless CRT
It is estimated that CRT non-responders account for approximately 30% of all implants. Occasionally, reversible causes for non-response can be found and addressed, such as suboptimal AV interval timings, inadequate biventricular pacing from ventricular ectopy or atrial arrhythmias, insufficient medical therapy or anaemia.
The wireless stimulation endocardially for cardiac resynchronisation (WiSE-CRT) system (EBR Systems) delivers ultrasonic energy to an LV endocardial receiver electrode to achieve biventricular pacing and may be a solution to patients who failed to have a conventional CRT implanted.
Endocardial LV pacing has some advantages over epicardial pacing and may offer an alternative in patients who do not respond to epicardial pacing. The technology eliminates the need for an LV lead and is designed to let the doctor place the stimulation point at an optimal, patient-specific location inside the LV, which could potentially be more effective.
The WiSE-CRT device consists of a tiny receiving electrode implanted in the LV, an ultrasound transmitter, which is implanted surgically in an acoustic window (typically in the fifth or sixth intercostal space) after screening by a physician to keep continuous communication between the LV electrode and transmitter unaffected by the patient’s position or inhale/exhale, and a battery pack.26 The electrode receives a synchronised ultrasound signal from the small transmitter with every heartbeat. The sound waves are converted to electrical energy, providing cardiac pacing. The WiSE-CRT system has been shown to reliably produce biventricular pacing.
A study by Sieniewicz et al. to assess the safety and efficacy of the WiSE-CRT system during real-world clinical use showed that LV endocardial pacing with the WiSE-CRT system is technically feasible with a high success rate.27 However, three procedural deaths occurred during the study which involved 90 patients. This means that procedural complications mandate adequate operator training and implantation at centres with immediately available cardiothoracic and vascular surgical support.27
Results from phase I of the SOLVE CRT study confirmed the high success rate of LV endocardial electrode placement with favourable clinical responses in heart failure symptoms.28 There were three device-related complications in a total of 31 patients.
A totally leadless biventricular pacing system has been recently described using a combination of Micra and WiSE-CRT systems.29 Leadless biventricular pacing may be particularly attractive for patients with leadless pacemakers who develop iatrogenic dyssynchrony-related LV dysfunction.29 However, this technology has several limitations. It remains unclear what happens if the wireless intracardiac electrodes reach the end of their life, as extraction of these devices could be complicated due to advanced endothelialisation.
The high cost of the WiSE-CRT device is still an issue. Considering the two-step implantation approach, the periprocedural and device costs may be higher than that of conventional CRT systems.
Lastly, energy transmission via ultrasound is less efficient than via conventional wires. This leads to a more frequent need for battery replacement.
Battery-free Pacing
Scientists have successfully tested a heartbeat-powered pacemaker in living pigs.30 Researchers say this is an important step toward developing battery-free implantable medical devices. The new ‘symbiotic pacemaker’ consists of three components: a wafer-sized generator attached to the heart that converts the organ’s mechanical energy into electrical energy; a power management unit that has a capacitor to store that energy; and the pacemaker itself, which stimulates and regulates the heart muscle.
The study results are encouraging, but there is a lot of work to be done before it might be used in humans. The energy harvest device would need to be inserted around the heart in open heart surgery, which is a lot more invasive than is needed for current pacemakers and would greatly limit candidates for the pacemaker. However, the device could use movement from other muscles rather than the heart, so this should not be a problem. People who need the pacemaker to work a lot or to deliver defibrillation use a lot more electricity, so it is important that any self-powering device has enough stored energy for these situations.
Biological pacemakers modify non-pacemaker myocytes to provide automaticity using gene therapy technologies or add pacemaker syncytia to the heart through adult or embryonic stem cell therapies.31 Biological pacemakers are in the early stages of development, and current challenges include the difficulty of ensuring long-term engraftment and the potential for proarrhythmia.
Conclusion
The field of cardiac devices has developed enormously since the 1950s and it continues to progress and innovate to improve patient care. The advent of LPPMs and the future possibility of battery-less pacemakers has opened new frontiers. Further research is required before some of these technologies are safely and routinely used in clinical practice. We predict that leadless cardiac devices will see significant growth and development over the next decade.