Transvenous Pacemakers
The prevalence of pacemaker implantation is rising globally with over one million implantations annually.1 An ageing population combined with an expanding array of indications is projected to fuel pacing demand further.
The transvenous pacemaker consists of a small generator implanted subcutaneously that is connected to electrical leads that fix to the cardiac chambers. The generator contains a battery, circuitry and capacitors; the leads contain conduction coils with layers of insulation. Electrical impulses from the generator travel via the leads to stimulate the heart; although the system is effective, up to 9.5% of patients develop complications often related to the lead(s) or the subcutaneous pocket housing the generator.2 Most of these complications are pocket haematomas or lead displacements, issues that create inconvenience but can be corrected with minimum risk.
Current transvenous systems are the culmination of more than 65 years of technological progress and clinical experience; generations of physicians have become experts in managing patients with these systems over many decades.3 This includes the routine use of devices that combine pacing with defibrillation and cardiac resynchronisation, including by the selective stimulation of the cardiac conduction system.
Leadless Pacemakers
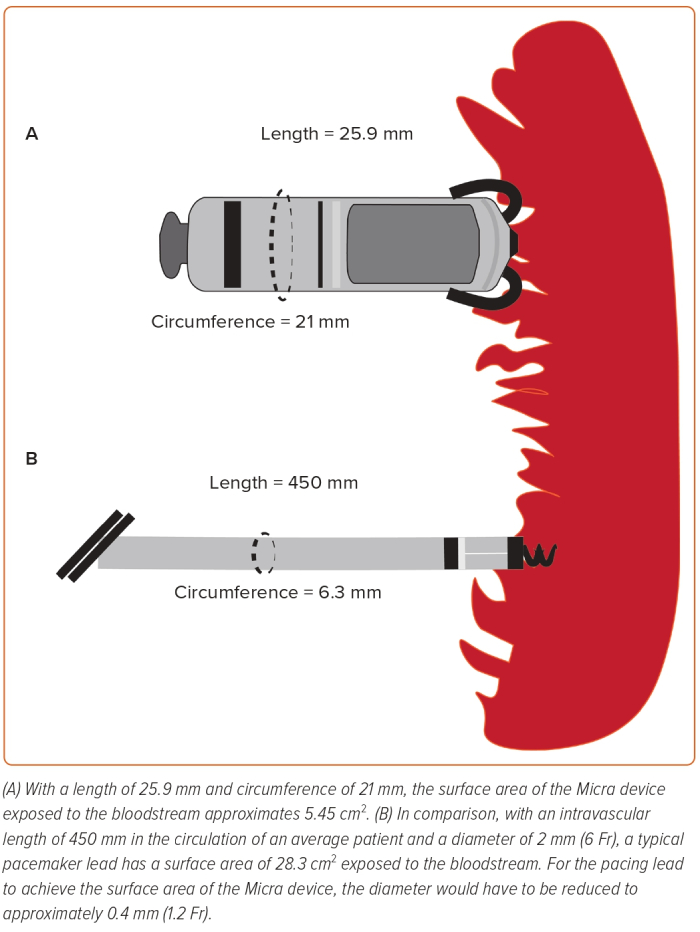
The leadless pacemaker (LPM) was conceived to overcome several limitations of the transvenous system through its self-contained, leadless design. This has been well summarised by Dilaveris et al.4 The LPM is associated with a significantly lower risk of infection than the transvenous system, probably because of a smaller surface area; the intravascular component of the LPM has a smaller surface area than that of a single pacing lead (Figure 1). The leadless design also eliminates the need for a subcutaneous pocket, which contributes to up to 60% of device infections, and reduces the risk of device colonisation by organisms seeded through the bloodstream.5 The leadless nature of the device is advantageous in patients lacking suitable access, including those who require renal replacement therapy. Delivery of the device (in experienced hands) is also less arduous than the implantation of a conventional system, with fewer steps and less requirement for surgical expertise. The absence of a generator pocket confers a cosmetic advantage, and without leads there can be no need for lead extraction, a procedure associated with greater risk than device implantation.
With evolution, new challenges arise. The atrioventricular (AV) synchrony in LPM remains inferior to that in conventional systems, so the leadless system is best suited to patients requiring VVI pacing or a low pacing burden.6 Patients with complete heart block benefit from dual chamber pacing over single chamber pacing due to the AV synchrony; atrial systole contributes to the left ventricle (LV) filling.7 The Micra AV algorithm attempts to address this limitation, but is not considered equal to the transvenous pacemaker.4
Management of the LPM following battery depletion is a concern; current practice dictates that the LPM should be abandoned and an additional LPM implanted. In a young patient who may require multiple implants across decades of pacing therapy, the abandonment strategy may result in a crowded right ventricle (RV), and it is unclear whether this will affect RV haemodynamics. Without evidence to demonstrate the long-term safety of multiple depleted devices in a human heart, abandonment is an approach of unknown risk. Extraction of conventional leads is an imperfect science, but it is safe and effective in a large majority of cases.8 Extraction of the leadless device is not yet routine, and there is no experience of extracting devices after more than a decade of use.
The implantation procedure also carries risk: the large-bore sheath housing the device increases the risk of cardiovascular injury, including tricuspid valve malfunction, cardiac perforation and death, whereas the small pacing device can embolise if the tines have not effectively hooked the myocardium. Traditional pacing systems carry an elevated rate of complications, but most of these are not life-threatening. The leadless pacemaker carries a lower total risk of complications, but the relative risk of a fatal complication has not been determined.
Leadless CRT
The leadless concept has been extended to CRT with the WiSE-CRT system. An endocardial LV electrode receiving signals from a subcutaneous ultrasound transmitter activates the LV in synchrony with the RV; the functioning of the system relies on a co-device situated in the RV. The leadless nature is beneficial because it overcomes the challenges of implanting the LV lead in the coronary venous system and removing restrictions on the implant site. When used in conjunction with the LPM, the CRT system can be fully leadless.
Again, this advance introduces new challenges. There are multiple procedures involved: the subcutaneous component is implanted separately to the LV electrode, translating into two separate procedures in addition to the procedure for implanting the co-device in the RV. Each procedure is associated with a significant risk of complications, including vascular injury, infection, perforation, embolisation and mortality.4,9 This is unfavourable compared with conventional CRT systems, which can be successfully implanted in up to 97–99% of cases (in experienced hands) on the first attempt, and in >99% of cases using unconventional methodologies with repeat procedures.10–12 The CRT implantation failure rate is extremely low.
The siting of the LV electrode and subcutaneous transmitter is also limited by an acoustic window; a clear intercostal path is required for the ultrasound to travel from the transmitter to the electrode. This can potentially limit the positioning of the LV electrode, which, therefore, cannot always be positioned in the optimal LV segment. Contrary to Dilaveris et al., we do not believe the leadless CRT system can be labelled as a suitable alternative for ‘non-responders’, at least not in a generalised sense.4 Non-response to CRT is a complex phenomenon with suboptimal AV timing at its core.13 Suboptimal LV lead positioning constitutes a relatively low proportion of ‘non-response’ and can often be overcome with the newer multipole leads, which multiply the number of pacing vectors available, potentially allowing programmed solutions to suboptimal lead siting.
Transvenous CRT systems have a major advantage: through their proprietary algorithms they have the potential to optimise AV timings, a need that itself is dynamic. These adaptive algorithms provide a continuous state of optimisation while attempting to fuse intrinsic RV depolarisation with LV pacing. The goal is to minimise RV pacing while constantly optimising the AV and interventricular timings because they are not static.14 The WiSE-CRT system has no such algorithm, and the imperfect AV tracking by leadless devices is likely to be more detrimental to the CRT patient than in the general pacing population.
Costs should not dictate healthcare, but must be considered: a totally leadless CRT pacemaker would demand approximately £23,100, far higher than the £3,900 for a modest transvenous system; the addition of a subcutaneous ICD could push costs near to £40,000, whereas a transvenous CRT defibrillator can be obtained for close to £12,500. Perhaps due to the novelty of the system, the WiSE-CRT projected battery longevity of 4.5 years is difficult to achieve.15 The cost of device therapy accrues over time, so a comparison of costs must take durability into account. Doing so magnifies the disparity in up-front costs.
Conclusion
The leadless pacing system already plays a crucial role in a small group of patients. It has the potential for much more widespread usage, but it is too early for it to be declared a superior therapy to the transvenous system. Transvenous systems have taken more than six decades to reach their current level of sophistication and reliability. Significant limitations, including cost, must be overcome before leadless systems can challenge them across the spectrum.