Primary percutaneous coronary intervention (PCI) is the standard of care for the treatment for acute ST-elevation MI (STEMI) because it improves the prognosis by salvaging the jeopardised myocardium.1,2 However, in acute situations there is a high load of thrombus in the infarct-related artery and the coronary vascular resistance is also high. Stent placement in such a case enhances the chances of the thrombus shifting both proximally and distally in the microvasculature. The occlusion of microvasculature by thrombus leads to the no-reflow phenomenon.3
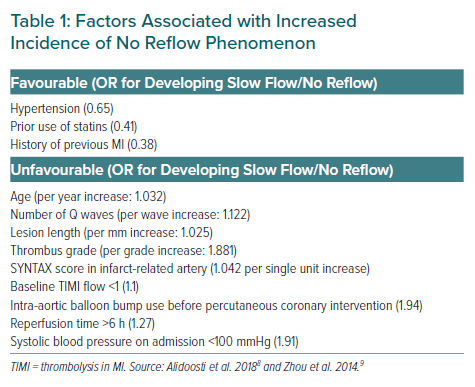
The pathophysiology of no reflow revolves around distal embolisation of clot, microvascular spasm and thrombosis.3 No reflow is seen in about 10% of cases of primary PCI and the predisposing factors include advanced age and delayed presentation and a completely occluded culprit artery with heavy thrombus burden.3–7 Table 1 lists the various clinical factors that predispose to the slow-flow/no-reflow phenomena.8,9 The no-reflow phenomenon leads to increased morbidity and mortality thus negating some of the benefits of PCI.4,7 Thrombus aspiration is an important adjunctive treatment but failure in randomised controlled trials (RCTs) led to guidelines advising against its routine use.10,11
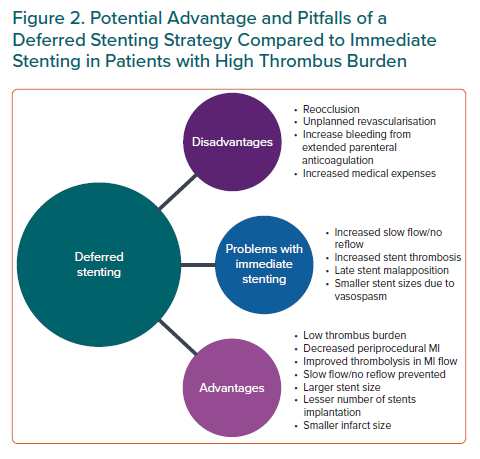
Deferred stenting is a novel strategy that aims to postpone stent placement for a fixed time window after stable distal flow has been achieved. The period of deferment allows gradual resorption of thrombus, improvement in thrombolysis in MI (TIMI) flow, decrease in vasospasm and decreased periprocedural complications of slow flow. Further, not infrequently the stent placement may be deferred altogether. The need for two procedures leads to prolonged hospitalisation and may add to the cost of the treatment, but the improvement of periprocedural outcomes and obviation of the need for a coronary stent can justify the financial burden. Hence, further studies are needed to elucidate whether the short-term increase in medical expenses by using the deferred PCI strategy is cost effective by improving long-term prognosis and reducing social expenses.
We report the case of successful deferred stenting in a 45-year-old man with STEMI and high thrombus burden. The deferred angiogram revealed significant attenuation of thrombus burden and improved TIMI flow. We further discuss the various trials, registries and meta-analyses reporting on the strategy.
Case Report
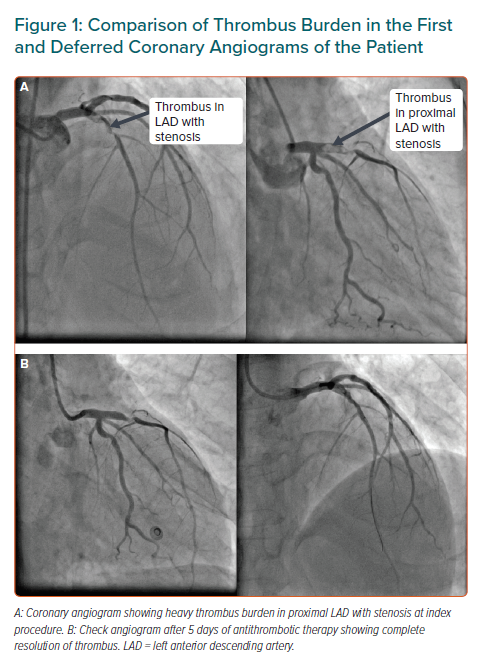
A 45-year-old man presented to the emergency department with acute anterior-wall MI of 8 hours duration. The patient had a history of hypertension and was a smoker. His echocardiogram showed hypokinesis in the left anterior descending (LAD) artery with an ejection fraction of 38%. The patient was thrombolysed and taken up for pharmaco-invasive PCI. His coronary angiogram showed a significant stenosis with grade IV thrombus in proximal LAD with TIMI 2 flow (Figure 1A). Due to high thrombus burden, stenting was deferred and the patient was put on IV eptifibatide (a glycoprotein IIb IIIa inhibitor) infusion for 18 hours followed by subcutaneous low molecular weight heparin twice daily.
Rescue PCI was planned in case the patient developed chest pain. After 5 days, his angiogram showed moderate stenosis and the thrombus in LAD was almost absent with improved TIMI 3 flow (Figure 1B). Since there was a significant obstruction in LAD, a direct stenting procedure with a 3 × 24 mm everolimus-eluting stent was performed and the results were optimised by post-dilation with a non-compliant balloon. The end result obtained was a TIMI 3 flow without any residual stenosis or complications.
Discussion and Literature Review
Primary PCI within the window period of 12 hours of symptom onset is the standard of care for patients with STEMI.1,2 However, in a fraction of these patients reduced coronary blood flow (slow flow or no reflow) is seen despite epicardial vessel patency with PCI, and this is associated with a worse prognosis.3,12 Distal migration of thrombus and atherosclerotic debris are important contributors to interventional slow flow/no reflow. Thrombus removal with thrombectomy devices has been found to be useful, but RCTs of mechanical and manual thrombectomy and distal protection have not shown consistent benefits and, an association with stroke was observed in some trials.13–16 Presence of residual thrombus even after manual aspiration is one of the pitfalls and it predicts poor outcomes.17,18 Thrombus grading on angiography is done by the Gibson’s angiographic score/TIMI criteria.19 A thrombus grade higher than 3 is usually considered as high thrombus burden.
Deferred Stenting Strategy: A Primer
The strategy of deferred stenting represents a radical change in the management of patients with STEMI during PCI especially if they have a high thrombus burden. The stent placement is delayed by a finite interval after the index procedure when stable distal flow has been restored by PCI or thrombolysis. This time of deferment has multiple benefits – gradual clearing of the thrombus, improvement of microvascular flow, reduction of vasospasm, prevention of distal embolisation, avoidance of slow flow/no reflow and attenuated periprocedural MIs. Indeed, data suggests the coexistence of thrombus and spasm and hence a deferred strategy can lead to better stent selection (large and short stents).20,21 In some cases, it can lead to deferment of stent placement altogether. Moreover, placing a stent in an artery with high thrombus burden can lead to malapposition and increased stent thrombosis as documented in imaging studies, probably due to selection of smaller size stents and longer devices.22,23
On the other hand, there is a possible risk of reocclusion during the waiting period which can be mitigated by parenteral anticoagulants and glycoprotein (GP) IIb/IIIa inhibitors. A rescue PCI should be considered if necessary. A prolonged systemic anticoagulation can increase the risk of bleeding which can be detrimental. However, the use of the CRUSADE bleeding risk score can help to assess the baseline bleeding risk of the patient. It has been validated in a cohort of nearly 18,000 patients and scores <20 indicate a very low risk of in-hospital bleeding.24 The use of GPIIb/IIIa inhibitor infusion should be avoided in patients who have a high CRUSADE score (>20) to mitigate the bleeding risk from prolonged anticoagulation (intracoronary bolus may be considered in high-risk patients). Patients with a score >50 are at a very high risk of bleeding and the duration of low-molecular-weight duration should be reduced to 72 hours instead of the standard proposed duration of 5–7 days.
Another pitfall with deferred PCI is the need for two procedures that will lead to prolonged hospitalisation, as well as adding to the cost of the treatment. However, the improvement of periprocedural outcomes and obviation of the need for a coronary stent can justify the financial burden. This calls for further studies to evaluate the short-term increase in medical expenses versus the improved long-term prognosis and whether this will translate into reduced care costs during follow-up. Figure 2 summarises the key advantages and pitfalls of a deferred strategy versus an immediate stenting approach.
Other terminology used in the literature for a procrastinated stenting approach include delayed PCI and secondary PCI.25 Minimally invasive mechanical intervention (MIMI) is an adjuvant technique during primary PCI before deferring stent placement in arteries with TIMI 0–1 flow.26 The strategy entails the use of a guidewire, an undersized balloon catheter and thrombus aspiration to establish distal coronary flow.27 The aim is to restore the flow with minimal forward propagation of thrombus. In the following sections, we discuss the available data regarding this approach.
Single Centre and Non-randomised Studies
Initial registries and non-randomised data (before 2010) suggested that a strategy of delayed or deferred stenting, when stable coronary blood flow has been achieved in the infarct-related artery reduces the risk of embolisation and thereby potentially improves the clinical outcome.28–30
Data accumulated in the past decade has reaffirmed that deferred stenting reduces thrombus burden, improves microvascular flow, increases myocardial salvage, improves left ventricular ejection fraction (LVEF) and attenuates major adverse cardiac events (MACE) in patients with STEMI.31–34 Male sex, younger age, larger size of culprit artery and higher thrombus burden at baseline were predictors of greater benefit from deferred stenting.33 It is also interesting to note that association between stent implantation and improved outcomes has not been very consistent.35,36
In a Danish pilot study, the need for subsequent stenting was reduced by 38% without any risk of reocclusion at 3 months with a deferred strategy.37 In a previous study by Ke et al., subsequent stents were avoided in 23% of patients.32
In a French study, Souteyrand et al. used optic coherence tomography (OCT) to guide deferred stenting.38 The study tested the safety of three different strategies – acute (<2 days), early (up to 7 days) and late deferral (up to 1 month) in the setting of STEMI with large thrombus burden on angiogram. There were no MACE recorded between initial and final procedure at a mean of 9 days. The thrombus presence as assessed by OCT continued to diminish from acute phase (94.1%) to early phase (78%) to late phase (32%). Not only presence of thrombus but also the length, score and area of thrombus continued to decline on OCT from acute to late groups. This study demonstrated that OCT-guided postponement of stent implantation led to good procedural outcomes with 100% success and alleviation of no-reflow events. This is the first imaging-guided evidence for the continuum of resorption of thrombus on prolonged deferral until a week and beyond up to 30 days.
Building upon the results of previous studies, the SUPER-MIMI study tested a longer deferral time of 7 days in 155 patients with STEMI.39 There was an improvement in TIMI flow, decrease in thrombus burden and stenosis severity diminished. More importantly, stenting was also avoided in 38% cases with a minimal chance of reocclusion (1.3%). Studies have not only used metallic drug-eluting stents (DES), but also bioresorbable stent (BRS), with Combaret et al. demonstrating successful deferred implantation of BRS in 45 patients with STEMI under OCT guidance between 2 and 7 days. 40 TIMI flow improved and thrombus burden declined on subsequent angiograms. BRS implantation was altogether avoided in 25% cases and MACE rate was low at 6 months.40
Randomised Controlled Trials
DEFER STEMI
In the DEFER-STEMI study, patients were randomised to either conventional stenting or deferred stenting with an intent to implant stent after prolonged antithrombotic therapy after an initial achievement of TIMI 2–3 flow.41 Patients with STEMI along with angiographic or clinical features for risk of slow flow/no reflow were enrolled for the study. There was significant reduction in incidence of the primary endpoint of slow flow/no reflow in the deferred group (5.9% versus 28.6%, OR -0.16; p=0.005). There were also fewer thrombotic events during PCI while the final TIMI flow was higher. The amount of myocardium salvaged on cardiac MRI was higher in the deferred stenting group on long-term follow-up and the percentage of salvaged myocardium is one of the prognostic indicators in STEMI PCI. Also, a larger percentage of patients achieved greater stent diameter in the deferred strategy group.
DANAMI 3-DEFER
On the contrary, the DANAMI 3-DEFER trial failed to show any benefit of deferred stenting on clinical outcomes.42 About 1,200 patients were randomised to a deferred stent strategy versus an immediate stenting technique. There was no significant difference in the primary outcome (composite of all-cause death, hospitalisation for heart failure, repeat MI and unplanned revascularisation; 7% versus 18%; p=0.92). In addition, there was a slightly higher, although not significant, chance of reocclusion rates (2%) in the deferred stenting group. However, there was an insignificant improvement in LVEF in the deferred stenting group, but whether that translates into better clinical outcomes is not known. An MRI sub-study also failed to find any benefit on myocardial infarct size, microvascular obstruction and myocardial salvage index.43 However, in patients with lesion length/stent >24 mm, the deferred strategy significantly reduced infarct size. This finding has been corroborated in other studies.21
Comparing Randomised Controlled Trials
Why are there contrasting results from two large RCTs on deferred PCI? First, the DEFER STEMI enrolled patients at high risk of slow flow based on clinical angiographic features, whereas DANAMI 3-DEFER was an all-comer primary PCI study (Supplementary Material Table 1). A deferral strategy should only be applied after careful angiographic selection.
Second, DEFER STEMI was an angiographic and MRI endpoint study whereas DANAMI 3-DEFER looked at clinical outcomes. We know that clinical outcomes are affected by many variables and imaging features are only one of the facets. We can conclude that the benefits of reducing slow flow in some high-risk patients could have been counterbalanced by reocclusions due to unnecessary deferral in low-risk patients.
Third, DANAMI 3-DEFER was a larger, multicentre, randomised study, in contrast to DEFER, which was a small, single-centre, proof of concept study. The analogy is similar to renal denervation studies where data from the small SYMPLICTY 1 and 2 studies failed to translate into benefit in the large SYMPLICTY 3 study.44
Fourth, the use of GPIIb/IIIa inhibitors in DANAMI 3-DEFER was significantly lower compared to DEFER STEMI (35% versus 98%). The duration of infusion was also lower (at least 4 hours versus 12 hours). Robust parenteral anticoagulant and antithrombotic agents are integral components of a defer strategy, as discussed earlier. In fact, the majority of patients in the DANAMI 3-DEFER study received bivalirudin which is no longer recommended during PCI because of high rates of stent thrombosis.45 These factors could be the major contributors to high reocclusion rates seen and attenuation of clinical benefits in the deferred arm.
Finally, there was high crossover to immediate stenting (22%) in the defer arm of the DANAMI trial which further weakened the results.
Recent Trials
In the MIMI trial, deferred stenting at a median 36 hours was studied in 140 patients with STEMI.46 Microvascular obstruction on cardiac MRI at 5 days was non-significantly lower in the immediate stenting group.
The INNOVATION study did not find any merit in a routine defer strategy during primary PCI at two centres in South Korea.47 In the subset of anterior infarction, the primary endpoint – infarct size and microvascular obstruction – was significantly attenuated.
PRIMACY is the latest RCT in the series, with results presented at the European Society of Cardiology congress in 2019. The study randomised 305 STEMI patients to immediate versus delayed stenting. Only demographic and angiographic characteristics were reported separately and the outcomes of the study were reported as pooled data of 1,873 subjects after combining data from the four RCTs discussed above (DEFER STEMI, DANAMI 3-DEFER, MIMI and INNOVATION).48 The data from PRIMACY was treated as exchangeable with the four other RCTs and a patient-level data meta-analysis was used with hierarchical predefined distribution of the risk ratio.49 There was adequate use of GPIIb/IIIa inhibitors (80–90%) and the mean delay in stenting was 42 hours. The slow-flow/no-reflow rates were reduced in the deferred arm (24% versus 27%). Distal embolisation was also lower in the deferred arm as was the composite of adverse angiographic events. The combined endpoint of cardiovascular death, MI, heart failure and urgent target vessel revascularisation (TVR) in the pooled analysis was not different in either of the arms. However, a deferred strategy led to reduction in cardiovascular death and heart failure in the long term, which was counterbalanced with increased rates of unplanned TVR. Full data from the meta-analysis is yet to be published.
Meta-analysis Data
A meta-analysis by Freixa et al., which encompasses six studies (five non-randomised) demonstrated that deferred stenting was safe with only three coronary reocclusions occurring among 283 patients and beneficial as it improved left ventricular function with a lower MACE rate.50 The main criticism of the meta-analysis is the exclusion of all the RCTs discussed above. The only RCT included enrolled patients with non-STEMI and assessed the role of early versus delayed invasive PCI unrelated to thrombus or slow flow.
Subsequently, Qiao et al. in their meta-analyses of nine studies (three RCTs and six observation studies) found no difference in incidence of slow flow/no reflow overall.51 But observational studies showed a significant decline in its incidence (OR 0.12; p=0.002). Deferred stenting conferred an advantage in terms of improvement in LVEF in the long term but no difference in MACE.
Most recently, Cassese et al. in a collaborative meta-analysis of all four RCTs of deferred stenting with MRI data, concluded that baseline thrombus burden (>grade 3) and longer stent length correlated with slow flow and microvascular obstruction.52 Deferred stenting potentially improved angiographic outcomes however, this did not translate into clinical and imaging outcomes.
What Should Be the Ideal Deferral Time?
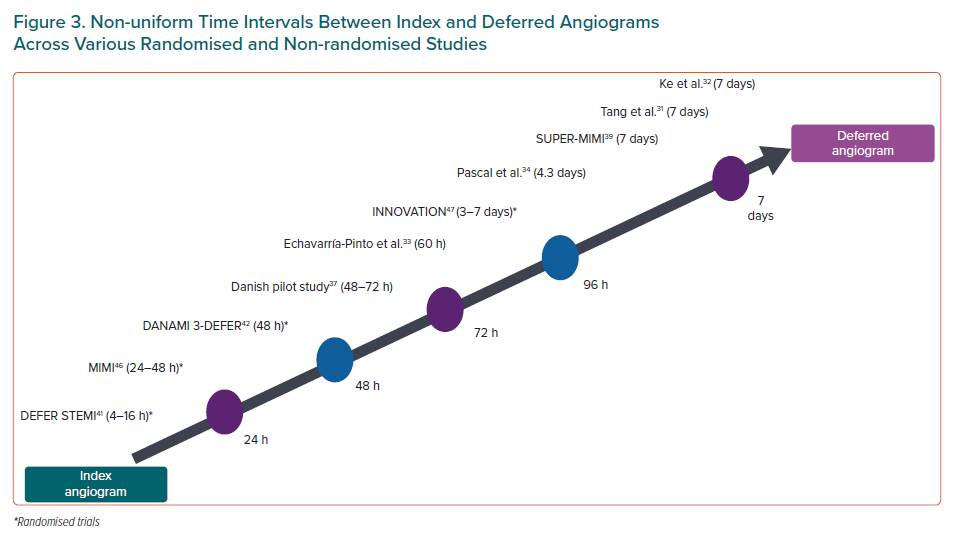
The studies discussed have used variable time intervals between the index angiogram and the deferred angiogram (Figure 3). The usual time elapsed is between 24–72 hours in the RCTs except INNOVATION, which used a time lag of 3–7 days. Similarly, the majority of the 10 non-randomised studies also used a time delay between 24–72 hours. Only four studies have used a longer 6–7-day window period for deferral.28,32,33,40 Many researchers, including our group, consider the 24–48-hour window period too short for meaningful thrombus resorption and to register effective action of antithrombotic agents.53
The French study clearly demonstrated persistent thrombus resolution with time delay up to 30 days. There was also increase in arterial diameter, decrease in stenosis diameter and improved TIMI flow with time too. Although appealing, a 1-month deferral is not practical as it leads to a prolonged hospital stay, challenges in parenteral anticoagulation and a financial burden on the health system. A 7-day deferral is plausible with additional thrombus attenuation and improved periprocedural advantages though only a minority of RCTs enrolled such patients. Moreover, to achieve the optimum benefits of the defer strategy, we believe a deferral time of 5–7 days is needed in patients with a high thrombus burden.
At our institution, we administer IV GPIIb/IIIa inhibitor for 12–16 hours after the procedure followed by low molecular weight heparin for 5–7 days or until the next angiogram depending upon the baseline CRUSADE score. In patients with a CRUSADE score >20, the GP IIb/IIIa infusion is avoided and only intracoronary bolus is provided. For those at very high risk of bleeding (score >50), the duration of heparin is also reduced. This protocol minimises the chances of reocclusion and bleeding events are also reduced. A rescue PCI should be performed whenever needed.
How to Select Patients for Deferred Treatment
A uniform approach was missing among the RCTs and non-randomised studies (Supplementary Material Table 2). While the DEFER STEMI trial selected patients based on one or more angiographic feature of slow flow/no flow, the other four RCTs were all-comer primary PCI studies with TIMI 2–3 flow achieved after a MIMI strategy. On the contrary, most single-centre observational and non-randomised registry studies recruited patients with high thrombus burden.27,30–32,37–38 Moreover, two different definitions of high thrombus burden were used.19,54 Selection of such a high-risk group poised for adverse events with immediate PCI could have resulted in success in the majority of non-randomised studies while the majority of RCTs enrolling an all-comer population failed to demonstrate benefits. Indeed, large thrombus burden is an ardent precursor for stent thrombosis and MACE with DES implantation in acute MI.55
The failure of DANAMI DEFER and other RCTs precludes a defer strategy for all STEMI patients. However, the success of the randomised and non-randomised studies that chose high-risk subjects favours such a strategy in routine practice. First, the presence of large thrombus burden on angiogram during PCI for STEMI or acute coronary syndrome (ACS) is the most widely accepted criteria for a defer strategy. In the setting of primary PCI, a MIMI strategy is highly recommended and was used in most RCTs. In case of a pharmacoinvasive PCI, the use of MIMI is optional and will depend upon the clinical scenario as well as operator preference.
Second, presence of long lesion/stent length >24 mm is an additional indication for a defer strategy. When neither of these are present, the presence of two or more clinical and non-clinical criteria predisposing to a high risk of slow flow could be the third criterion used to guide a defer strategy (Table 1). Younger age, male sex and large diameter of culprit vessel are other candidates who will do well with a defer strategy. Last, anterior infarction is another subset which purportedly attains benefit from a defer strategy, probably owing to the large area of myocardium at risk, warranting a low threshold to adopt a defer strategy in anterior wall MIs.
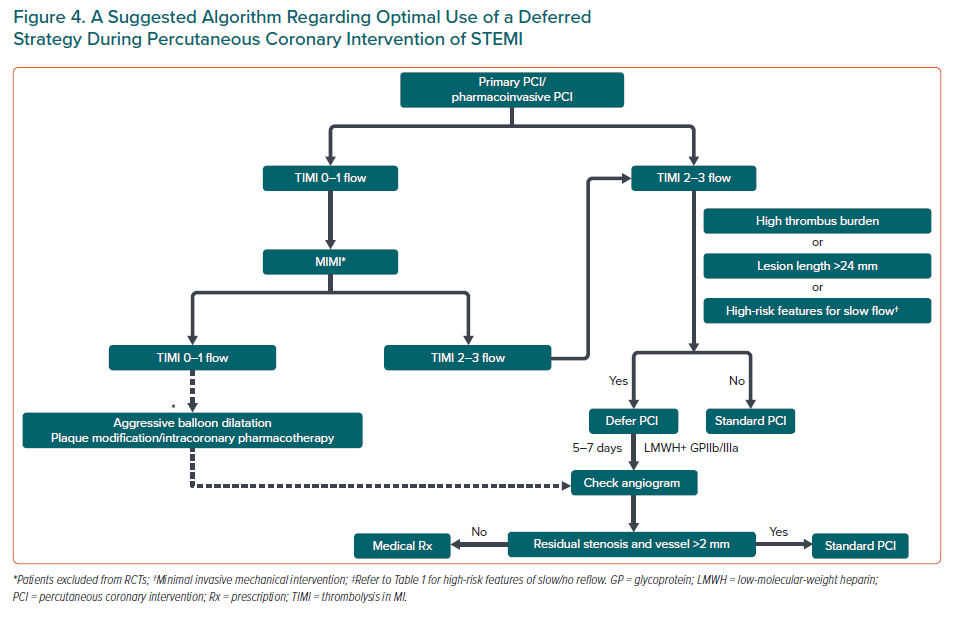
A suggested algorithm for optimal use of a defer strategy during PCI for STEMI/ACS is presented in Figure 4. We would like to emphasise that the prerequisite for implementing defer PCI is securing a TIMI 3 flow. In some situations, optimal TIMI flow may not be secured even after MIMI. Extensive microvasculature damage and inadequate treatment of culprit lesion are common mechanisms for failure to achieve adequate TIMI flow in such a scenario. Therefore, both of these situations require more intensive intervention than MIMI, rather than defer PCI. Uncommonly, even after aggressive lesion preparation and intracoronary pharmacotherapy, a TIMI 3 flow might not be feasible. Deferred PCI in such a scenario has not been tested in randomised trials but nevertheless the use of a coronary stent would also be questionable. Such patients represent a grey area and an individualised approach is needed.
Future Directions
Two future studies of defer stenting are underway to provide more answers – INNOVATIONCORE (NCT03744000) and OPTIMAL (NCT03282773). The publication of an updated meta-analysis of five RCTs including PRIMACY will also shed further light.
Conclusion
Heavy thrombus burden is not uncommon during PCI for STEMI and is an important contributor to the slow-flow /no-reflow phenomenon. Deferred stenting aims to lower thrombus burden and improve TIMI flow during the deferment time with the help of anticoagulants. Multiple studies and meta-analyses have shown benefits in terms of reduced slow flow/no reflow, improvement in ejection fraction and decreased MACE in some. A routine deferred strategy has not been shown to be beneficial and is definitely not advisable. But cases with high thrombus burden, longer lesions, high-risk features for slow flow and those with suboptimal TIMI flow (0–1) after thrombus aspiration can be candidates for deferred stenting. The ideal window for deferral is a matter of debate but longer deferral periods up to 5–7 days have proven to be safe and are advocated. Adjuvant parenteral anticoagulation is essential.
Click here to view Supplementary Material.