Transmitral valve repair can be performed with the MitraClip, a catheter-based percutaneous edge-to-edge repair technique to correct mitral regurgitation (MR), by connecting the anterior and the posterior leaflet of a regurgitant mitral valve. Data from the EVEREST II high-risk registry, as well as Asian registry data, demonstrate that the MitraClip procedure is feasible and safe.1–3
MitraClip was also evaluated in MITRA-FR and COAPT. Both these randomised controlled trials included patients with heart failure (New York Heart Association [NYHA] functional class ≥2 despite optimal guideline-directed medical therapy [GDMT]), reduced ejection fraction, and moderate-to-severe or severe secondary MR who received medical treatment with or without MitraClip implantation. The MITRA-FR trial did not show a significant difference in the composite primary endpoint of death from any cause and unplanned hospitalisation for heart failure at 12 months (54.6% in the device group versus 51.3% in the medical group; p=0.53).4 In contrast, the COAPT trial showed that the primary endpoint of all hospitalisations for heart failure within 24 months was significantly lower in the device group than in the control group (35.8% versus 67.9%, p<0.001).5 The 2020 American College of Cardiology/American Heart Association guideline for the management of patients with valvular heart disease recommends the MitraClip in treating severely symptomatic patients with primary MR who are at high or prohibitive surgical risk.6
In Asia-Pacific, MitraClip has been reserved for patients who are at high or prohibitive surgical risk, although many Asian patients with intermediate or low surgical risk still refuse to undergo surgery.3 For these patients, the MitraClip may be a reasonable treatment option. However, published data on the use of the MitraClip among Asian populations are limited compared with the West.7 The multicentre retrospective MARS registry, involving eight sites in five Asia-Pacific countries, reported the early experience in Asia with 142 patients who underwent the MitraClip procedure from February 2011 to October 2013. In this study, the acute procedural success rate was 93.7%.2
Given the limited published clinical evidence on the use of MitraClip in the Asia-Pacific region, the Asian Pacific Society of Cardiology (APSC) developed these consensus recommendations to provide expert guidance on the potential role of MitraClip in the treatment of MR in the region. These consensus recommendations are intended to guide general cardiologists and internists practicing cardiology in managing MR and evaluating patient suitability for MitraClip repair. However, the consensus recommendations are not intended to replace clinical judgement.
Methods
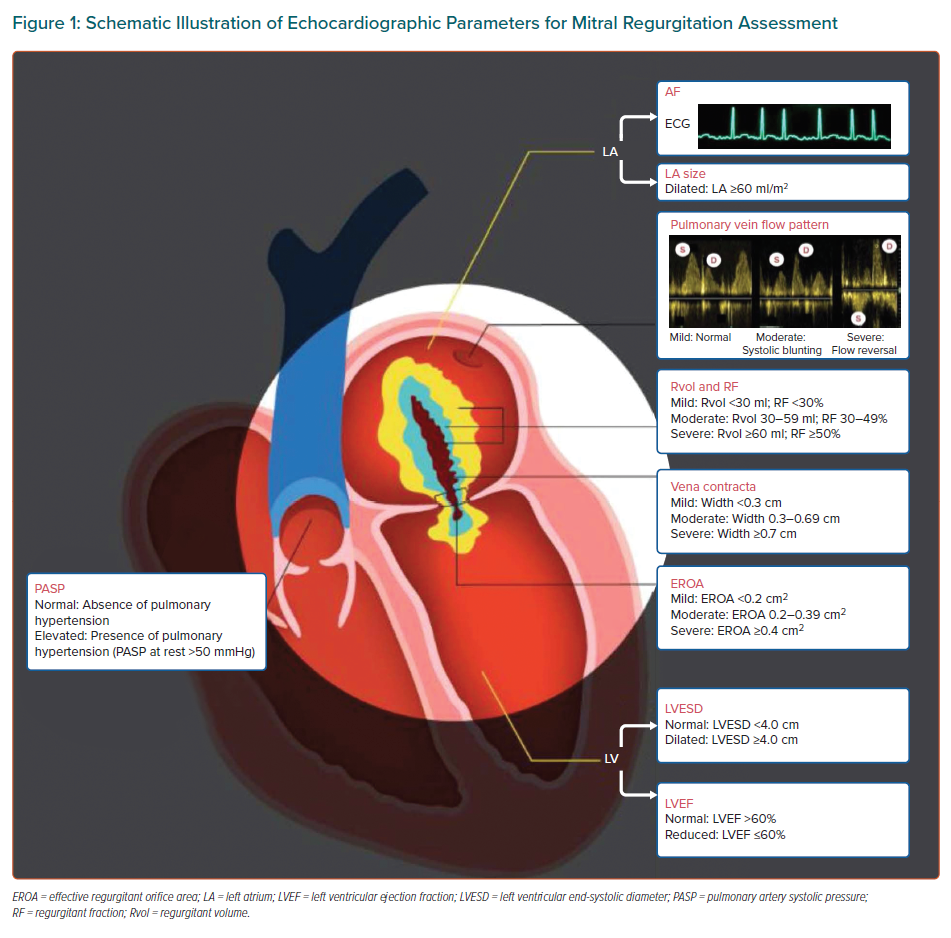
The APSC convened a 26-member panel to review the literature on the use of MitraClip in the management of MR, discuss gaps in the current management strategies, outline areas where further guidance is needed and, ultimately, develop consensus recommendations on the use of MitraClip. The experts were mostly members of the APSC who were nominated by national societies and endorsed by the APSC consensus board, as well as international experts in the MitraClip procedure. For these consensus recommendations, the expert panel decided to adapt the echocardiographic criteria from the American Society of Echocardiography guidelines, with a focus on parameters identifying severe MR patients suitable for MitraClip use (Figure 1), as the common definition for discussions during the consensus meeting.8
A comprehensive literature search was conducted, with particular focus on Asian-centric studies. Selected applicable articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system as follows:
- High (authors have high confidence that the true effect is similar to the estimated effect).
- Moderate (authors believe that the true effect is probably close to the estimated effect).
- Low (true effect might be markedly different from the estimated effect).
- Very low (true effect is probably markedly different from the estimated effect).9
The evidence was then discussed in two consensus meetings, held on 18 January 2020 and 27 June 2020.
Consensus recommendations for the use of MitraClip in the management of degenerative mitral regurgitation (DMR), functional mitral regurgitation (FMR), and other less common indications in the Asia-Pacific setting were developed during the two meetings. The statements were each put to an online vote using a three-point scale (agree, neutral, or disagree). Consensus was reached when 80% of experts voted agree or neutral. When there was not consensus, the statements were further discussed via email then revised accordingly until the criteria for consensus were reached.
MitraClip Use in Degenerative Mitral Regurgitation
Statement 1. Both symptomatic and asymptomatic patients with ≥3+ DMR, who meet the indications for surgery but are considered high risk by the Heart Team, should be considered for MitraClip implantation.
Level of evidence: Moderate.
Level of agreement: 80% agree, 16% neutral, 4% disagree.
Statement 2. MitraClip use should be considered for symptomatic high-risk ≥3+ DMR patients with or without reduced left ventricular ejection fraction (LVEF).
Level of evidence: Moderate.
Level of agreement: 84% agree, 12% neutral, 4% disagree.
Statement 3. MitraClip use should be considered for asymptomatic patients with high-risk ≥3+ DMR, with:
- Reduced LVEF and/or LV dilatation; or
- New onset AF or pulmonary hypertension.
Level of evidence: Low.
Level of agreement: 84% agree, 16% neutral, 0% disagree.
DMR, wherein one of the components of the mitral apparatus (leaflets, chords or papillary muscles) is affected, is often the result of degenerative mitral valve disease characterised by morphological changes in the connective tissue of the valve over time and resulting in MR.10,11 DMR may be either the result of fibroelastic deficiency or due to diffuse myxomatous disease.12,13 It may present across a spectrum ranging from isolated prolapse of a single leaflet scallop to bileaflet prolapse and annular dilation.10
Surgical mitral valve repair or replacement is still the gold standard for DMR, although transcatheter therapy may have a role in the management of a subset of patients.7,8,14,15 Data from MARS found that the acute procedural success rate for patients with DMR was 92%.3 The 30-day major adverse event rate was 14.7%, which was not significantly different from FMR patients (9.2%; p=0.555). Both FMR and DMR patients had significant improvements in the severity of MR and NYHA functional class after 30 days. There was a significantly greater reduction in LV end-diastolic diameter (p=0.002) and end-systolic diameter (p=0.017) in DMR than in FMR.
In addition, the AVJ-514 trial also provided Asian evidence to support the use of MitraClip in DMR patients. AVJ-514 was a prospective, multicentre, single-arm study that included patients with symptomatic chronic moderate-to-severe (3+) or severe (4+) DMR (n=16) or FMR (n=14). Among DMR patients, the acute procedural success rate was 87.5% and 81.3% had MR grade ≤2+ at 30 days.16 The proportion of patients with NYHA functional class III/IV was reduced from 37.5% to 6.3%. No deaths were reported.
The panel agreed to recommend MitraClip for high-risk patients with symptomatic severe DMR with or without reduced LVEF; high-risk asymptomatic patients with severe DMR with reduced LVEF and LV dilatation; and those with new-onset AF or pulmonary hypertension, who meet the indications for surgery but are considered high risk by the Heart Team. However, some panellists underscored the absence of clinical data in asymptomatic patients, which then lowered the level of evidence for this subgroup. One dissenting opinion for Statement 1 explained that only asymptomatic patients with prohibitively high risk should be considered for MitraClip therapy. For Statement 2, one dissenting opinion explained that such patients should be considered for surgery first, and MitraClip should be limited to those with high surgical risk.
While some experts stated their preference to treat early prior to deterioration or severe LV remodelling, the overall panel opinion was that there was no reason to intervene in asymptomatic patients with severe DMR with good LVEF and no LV dilatation (except those with new-onset AF or pulmonary hypertension). These patients should continue to be observed closely.
MitraClip Use in Functional Mitral Regurgitation
Statement 4. MitraClip should be considered for (≥3+) symptomatic FMR patients who are already on GDMT. FMR patients should receive at least 1 month of optimised GDMT, with reasonable attempts to uptitrate treatment, as well as cardiac resynchronisation therapy defibrillator (CRT-D) if indicated, before being evaluated for further intervention or MitraClip use.
Level of evidence: High.
Level of agreement: 88% agree, 8% neutral, 4% disagree.
Statement 5. For patients with ischaemic FMR (≥3+), coronary anatomy, ischaemia evaluation and potential revascularisation should be performed before MitraClip consideration. If the coronary revascularisation strategy is percutaneous coronary intervention, staged MitraClip should be considered. If the coronary revascularisation strategy is coronary artery bypass graft (CABG), concomitant surgical mitral valve repair/replacement may be considered.
Level of evidence: Low.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Statement 6. FMR patients should be monitored regularly (e.g. every 6 months) and referred early to the Heart Team (including a MitraClip specialist, heart failure specialist, echocardiologist and surgeon) for intervention, including MitraClip implantation. Discussions and endorsements of futility should be deferred to the Heart Team.
Level of evidence: Low.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Statement 7. Symptomatic patients with ≥3+ FMR should be assessed by the Heart Team for possible MitraClip implantation.
Level of evidence: High.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Statement 8. FMR patients who do not meet the eligibility criteria for MitraClip implantation (e.g. asymptomatic patients, those with MR severity of ≤2+, and those with less-optimised GDMT) should be closely monitored. These patients should be considered for MitraClip implantation once the eligibility criteria are met.
Level of evidence: Low.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
In FMR, the components of the mitral apparatus remain intact but there is resultant MR because of dysfunctional coaptation of the leaflets, usually as a result of ventricular or annular dilation.11 Patients with functional MR usually have LV dysfunction and most of them undergo medical treatment for the treatment of underlying conditions such as hypertension, dyslipidaemia, AF, coronary artery disease and heart failure. Guidelines recommend that these conditions be adequately treated – including the use of CRT-D when indicated – together with the management of the MR.14
Among those with FMR in Asia, 66% have an ischaemic aetiology.3 Hence, ischaemic evaluation is a crucial step in patient assessment. Coronary angiography is the preferred method of ischaemia assessment although the use of CT coronary angiography and other modalities would be acceptable in selected patients. Correctable ischaemia should be adequately treated (i.e. revascularisation and medical therapy); MitraClip may then be considered for severe symptomatic residual FMR (≥3+).
As first suggested by the EVEREST II trial, MitraClip is a suitable alternative treatment option for symptomatic FMR patients.1 The definitive COAPT trial also showed that patients with moderate-to-severe (grade 3+) or severe (grade 4+) secondary MR had a significantly lower rate of all hospitalisations for heart failure (primary endpoint) within 24 months with MitraClip compared with controls (35.8% versus 67.9%; p<0.001).5 All-cause mortality was also significantly lower in the device group (29.1% versus 46.1%; p<0.001). The secondary endpoints of quality of life, functional capacity, MR grade and LV remodelling also favoured MitraClip over controls.
The MITRA-FR trial did not show a significant difference in the composite primary endpoint of death from any cause and unplanned hospitalisation for heart failure at 12 months.4 On cursory examination, both the COAPT and MITRA-FR studies examined similar cohorts of FMR patients. However, there are several key differences in patient selection that have been instructive in the appropriate selection of the MitraClip and would also explain the differences between the two study outcomes. Firstly, the COAPT study is arguably a more robust study with a central inclusion committee, larger (almost double) sample size, detailed follow-up with echocardiography and functional studies (e.g. 6-minute walk test) and a requirement for stable doses of GDMT prior to enrolment, compared to the MITRA-FR. In addition, the COAPT study reported superior technical success rates and lower complication rates than the MITRA-FR study. Thirdly the criteria for MR severity also differed between COAPT and MITRA-FR, with the COAPT adopting more stringent criteria to qualify for severe MR. Finally, and perhaps most importantly, the COAPT study selected patients who had disproportionately severe MR compared to MITRA-FR patients who appeared to have more proportionate MR. The concept of disproportionate MR, first described by Grayburn et al. is beyond the scope of this paper, but in brief, refers to a situation where the MR is more severe relative to the LV volume.17 This implies a mechanical dysfunction of the mitral valve driving a significant element of the underlying heart failure.
According to the ACCESS-EU registry, which included patients with significant MR (77% of whom had FMR), there was an improvement in the severity of MR at 12 months compared with baseline (p<0.0001), with 78.9% of patients free from MR of >2+ severity at 12 months.18 The 6-minute-walk-test improved by 59.5 ± 112.4 m and the 1-year survival rate was 81.8%. The MARS registry also reported that the acute procedural success rates of MitraClip for FMR was 95.5%.3 Among the 14 patients in the AVJ-514 trial with FMR, acute procedural success rate was 85.7% and 92.9% of patients had MR grade ≤2+ after 30 days. The proportion of patients with NYHA functional class III/IV was reduced from 35.7% to 0.0%.16 No deaths were reported.
For patients with FMR, the panel recommended that MitraClip may be considered as long as medical therapy has already been dose-optimised for at least 1 month and, if appropriate, have had a CRT-D implanted. While a few panellists recommend observing on GDMT for up to 3 months, data from COAPT suggest that patients with more severe disease should probably not wait unnecessarily.
FMR patients who are potential candidates for MitraClip therapy should be monitored regularly by the general cardiologist via echocardiography, then referred to the Heart Team (MitraClip specialist, heart failure specialist, echocardiologist and surgeon) as soon as the patient fits the eligibility criteria (i.e. symptomatic moderate-to-severe FMR). All patients with LV dysfunction should undergo close surveillance.
The panel recommends the use of transoesophageal echocardiogram (TOE) to define MR aetiology while transthoracic echocardiogram (TTE) would be more appropriate for severity assessment as TOE may be influenced by haemodynamic variables during sedation. TOE may be used alone only for instances where the use of TTE is challenging.
Surgical risk should be considered by the Heart Team when deciding on the best treatment strategy, although surgical risk is not a predictor of MitraClip success or failure. Mitral valve surgery could be considered when concomitant CABG is required.14 In contrast, for severe FMR with LV dysfunction where no CABG is planned, MitraClip may be considered.
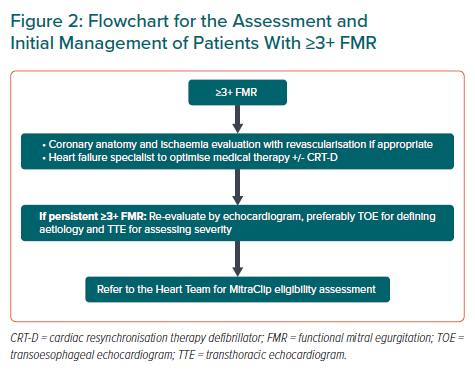
Figure 2 shows a flowchart to guide the assessment and initial management of patients with ≥3+ FMR.
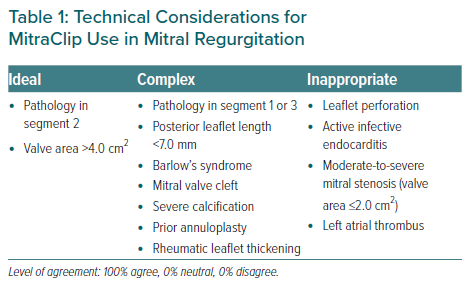
Additionally, the panel outlined the technical considerations for the use of MitraClip in the treatment of DMR and FMR (Table 1). The MitraClip currently has a fourth-generation ‘G4’ device, which has been enhanced with an expanded range of four clip sizes (NTR, NTW, XTR, XTW), differentiated by clip arm length and width, independent leaflet grasping feature and real-time left atrial (LA) pressure monitoring capabilities.19,20 The G4 system is already available in parts of Asia and these new features could aid in the treatment of challenging anatomies.
Subgroups and Special Populations for MitraClip Use
The use of the MitraClip was also considered in subgroups and special patient groups, including atrial FMR, concomitant MR/tricuspid regurgitation (TR), acute MR and hypertrophic obstructive cardiomyopathy (HOCM).
Statement 9. Patients with symptomatic atrial FMR should be evaluated by the Heart Team (including an electrophysiologist and heart failure specialist) and, if treatment has already been optimised, MitraClip may be considered.
Level of evidence: Low.
Level of agreement: 96% agree, 0% neutral, 4% disagree.
Although LA dilatation may occur in the absence of AF, a common cause of LA dilatation is long-standing AF.21 In patients with LA dilation due to AF, dilatation of the mitral annulus may lead to reduced leaflet coaptation and MR. Prominent LA dilation may cause posterior mitral leaflet tethering and restriction, which would result in atrial FMR.22
One study compared the effect of MitraClip therapy in AF patients with atrial FMR (n=38) with those with ventricular FMR (n=49). In this study, atrial FMR was defined as MR with preserved LV function (LVEF ≥50%) and normal LV wall motion, while ventricular FMR was defined as MR with LV dysfunction (LVEF<50%) or LV wall motion abnormality. The study found that MitraClip was associated with an improvement of MR, with an increase in leaflet coaptation and a greater reduction of anteroposterior diameter and mitral annular area in patients with atrial FMR than in ventricular FMR.23
Based on this study, the panel considered MitraClip a possible treatment option for symptomatic atrial FMR; hence, such patients should be evaluated by the Heart Team (including an electrophysiologist and heart failure specialist) for possible MitraClip implantation after other treatments have already been optimised. However, some panellists disagreed, pointing out that many patients with atrial FMR would be considered low risk for surgery, and maintained the role of surgical mitral valve repair in these patients. Nonetheless, for patients not amenable to surgery (e.g. some elderly patients), MitraClip is a reasonable option.
Lastly, it is worth noting that atrial FMR is often associated with severe TR24, which may limit the clinical benefit of transcatheter mitral intervention. Hence, this concomitant condition should also be assessed.
Statement 10. The expert panel acknowledges that MitraClip has been used in less common scenarios (e.g. acute MR, dynamic MR, HOCM, MR after failed surgical repair and TR) with reasonable reports of clinical success. However, enrolment into clinical trials or registries is preferred. Patients with these less common conditions should be evaluated by the Heart Team on a per-patient basis, with informed patient consent on the limited understanding available, to determine whether MitraClip use would be feasible and beneficial for them.
Level of evidence: Low.
Level of agreement: 100% agree, 0% neutral, 0% disagree.
Case reports have documented the use of MitraClip in less common scenarios, such as acute MR, dynamic MR, HOCM, MR after failed surgical repair and TR, with reasonable reports of clinical success.25–32
In the IREMMI trial, a multicentre registry that reported the feasibility of using MitraClip to treat acute MR in patients with acute myocardial infarction (n=93), patients had a procedural success rate of over 90% and a 30-day mortality rate of 6.5%.25
Another study, which included 221 patients who underwent MitraClip implantation, found that patients with dynamic severe MR experienced similar clinical improvement as patients with severe MR at rest, and that the majority of patients with dynamic severe MR experienced clinical improvement from NYHA functional class III/IV to I/II, as did those with severe MR at rest (59% versus 56%; p=0.566).26
For TR, one study of 64 patients with severe TR on optimal medical treatment, who were unsuitable for surgery and so were treated with MitraClip implantation, found that TR was reduced by at least 1 grade in 91% of the patients.31 Patients also had significant reductions in effective regurgitant orifice area (p<0.001), vena contracta width (p=0.001), and regurgitant volume (p<0.001). The 6-minute walking distance also increased significantly (p=0.007). A second observational study showed that in 50 patients, MitraClip treatment was associated with a 44% increase in 6-minute walk distance (p<0.001) and a non-significant 16% increase in quality-of-life scores (p=0.056).30 Despite these successes, clinicians should be aware that a separate catheter-based intervention (TriClip) has been evaluated in an international, prospective, single arm, multicentre study. This study, TRILUMINATE, found that in patients with moderate or greater TR, the TriClip reduced TR to moderate or less in 71% of patients (versus 8% at baseline; p<0.0001).33 Patients also experienced significant clinical improvements in NYHA functional class I/II (p<0.0001), 6-minute walk test (p=0.0023) and Kansas City Cardiomyopathy Questionnaire score (p<0.0001).
For HOCM, and MR after failed surgical repair, the current evidence has been limited to a few case reports.28,29,32 Beyond these reports, the use of MitraClip in these patients has not been evaluated in well-designed controlled trials so these patients should ideally be included in a clinical trial or patient registry. Should MitraClip be considered, patients with these less common conditions should be evaluated by the Heart Team on a per-patient basis, with informed patient consent on the limited understanding available, to determine whether MitraClip use would be feasible and beneficial for them. This opinion applies to patients who are not candidates for surgery, either due to surgical risk or due to lack of consent.
Conclusion
In all patients with MR, the aetiology, nature, and severity of the MR should be assessed, together with a thorough assessment of symptoms and overall surgical risk. Correctable underlying causes of MR should be adequately addressed together with the valvular problems. The determination of eligibility for MitraClip implantation requires a Heart Team approach to ensure a comprehensive benefit–risk assessment.