How we consider the natural history of coronary artery disease (CAD) was recently revised and updated in the 2019 European Society of Cardiology (ESC) guidelines for the diagnosis and management of chronic coronary syndromes.1 Chronic, progressive accumulation of atherosclerotic coronary artery plaques continues to occur during the seemingly clinically silent and ‘stable’ phases of patients between the index cardiovascular event and recurrent ones. Rather than ‘stable CAD’, this is more accurately defined as chronic coronary syndrome (CCS). This new definition subdivides CAD into acute and chronic phases with the trajectory of disease determined by coronary burden and comorbidities.
In the Asia-Pacific region, international guidelines cannot be routinely applied because of the unique characteristics of patients with CCS in this setting. These characteristics include differences in accesses and resources along with the low number of patients from the region in pivotal studies. Thus the Asian Pacific Society of Cardiology (APSC) developed consensus recommendations to provide guidance on the assessment and management of bleeding and ischaemic risks in Asian CCS patients. The selection of long-term antithrombotic treatment is also discussed.
Methods
The APSC assembled an expert consensus panel and convened two meetings (January 2020 and July 2020) to review the available evidence for antithrombotic management of CCS and discuss contemporary issues to provide guidance to clinicians in their local clinical context. The expert consensus panel comprised cardiologists, cardiac and vascular surgeons from Australia, Cambodia, Canada, France, Hong Kong, India, Indonesia, Japan, Korea, Malaysia, Singapore, Taiwan, Thailand, United Arab Emirates, UK and Vietnam. The experts were members of the APSC who were nominated by national societies and endorsed by the APSC consensus board.
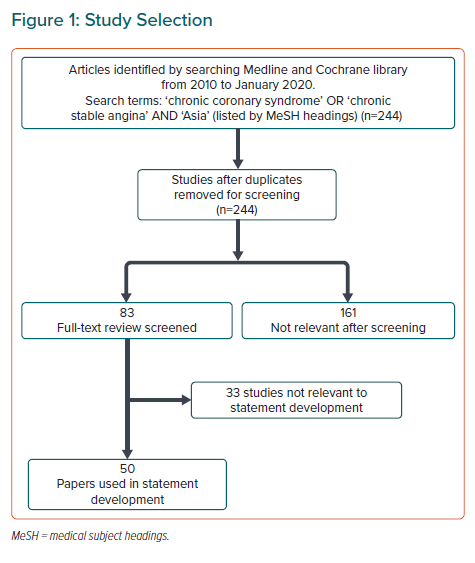
A comprehensive literature search was performed (Figure 1). Selected applicable articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system, as follows:
- High (authors have high confidence that the true effect is similar to the estimated effect).
- Moderate (authors believe that the true effect is probably close to the estimated effect).
- Low (true effect might be markedly different from the estimated effect).
- Very low (true effect is probably markedly different from the estimated effect).2
The available evidence was then discussed during two consensus meetings. Recommendations were developed during the meetings, which were then put to an online vote. Each statement was voted on by each panel member using a three-point scale (agree, neutral or disagree). Consensus was reached when 80% of votes for a statement were agree or neutral. In the case of non-consensus, the statements were further discussed via email, then revised accordingly until the criteria for consensus was reached.
Chronic Coronary Syndrome Diagnosis and Management Goals
Statement 1. Coronary angiography, cardiac CT or other imaging techniques may be used, where available, to confirm CCS diagnosis, estimate the extent of stress-induced ischaemia and evaluate burden of disease.
Level of evidence: Low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
In Asia Pacific, CCS is typically diagnosed based on the clinical evaluation of a patient with an accurate medical history and clinical testing. Where available and accessible, non-invasive coronary imaging may be used to detect and confirm a CCS diagnosis, allowing for the assessment of disease burden, visualisation of anatomical characteristics of disease, and determination of the individualised ischaemic risk to inform decision making.3 Patients who experience acute coronary syndrome (ACS) or who undergo percutaneous coronary intervention (PCI) transition to CCS management at the completion of their prescribed period of dual antiplatelet therapy (DAPT).
Statement 2. Concomitant patient risk factors should be appropriately managed according to local healthcare standards.
Level of evidence: Moderate.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
An important aspect of CCS management is to prevent thrombotic events. While antithrombotics include both antiplatelets and oral anticoagulants, antiplatelets, such as aspirin and P2Y12 inhibitors (clopidogrel, ticagrelor and prasugrel), are the most widely used for this purpose. Due to its lower cost and wide availability, aspirin was identified by the consensus panel as the antiplatelet of choice used for long-term antithrombotic management in the Asia-Pacific region.
The main concern with the use of antithrombotics is bleeding. Registry data as well as meta-analyses show that, compared with their white counterparts, East Asian patients have lower thrombotic risk, but a higher risk of bleeding, especially for intracranial haemorrhage and gastrointestinal bleeding.4–7 Thus, although international and regional guidelines recommend antithrombotics for the secondary prevention of thrombotic events, bleeding concerns have led to consistent underuse of antithrombotics in Asia.8,9
The focus of this document is to provide guidance for clinicians regarding the antithrombotic management and selection of treatments for CCS patients. We recognise that antithrombotic therapy is only one component of a comprehensive approach to management of these patients, which should include lifestyle changes, such as optimal nutrition, exercise and smoking cessation, along with risk factor management including abnormal blood sugar levels, hypertension, dyslipidaemia and obesity.10
Mitigating Thrombotic Risk Following Percutaneous Coronary Intervention
Statement 3. Imaging guidance, using intravascular ultrasound (IVUS) or optical coherence tomography (OCT), to optimise stent implantation is encouraged where available.
Level of evidence: Low.
Level of agreement: Agree 68.0%, neutral 32.0%, disagree 0%.
Statement 4. Drug-eluting stents (DES) should be preferred for PCI procedures where available.
Level of evidence: Moderate.
Level of agreement: Agree 96.0%, neutral 4.0%, disagree 0%.
Stent thrombosis is a major concern following PCI, with many patient and procedural factors contributing to the risk of stent thrombosis. While patient factors may be difficult to modify, specific procedural measures may be taken during PCI to mitigate thrombotic and/or bleeding risk and improve patient outcomes. The use of IVUS and OCT has been well-established for patient assessment prior to PCI, for lesion assessment, stent selection (diameter and length) and to optimise stent deployment.11 More recently, data have shown that the use of IVUS-guided stent implantation during PCI improves cardiovascular (CV) outcomes following the procedure, compared with angiography-guided stent implantation.11,12 In addition, the use of DES has also been associated with better outcomes and lower risk of stent thrombosis.13 Data from a nationwide study from Taiwan reported that patients receiving DES had better outcomes compared with those who had a bare metal stent implanted.14
Balancing Thrombotic and Bleeding Risk When Selecting Antithrombotic Therapy
Statement 5. The bleeding and thrombotic risk of a patient should be assessed before determining which antithrombotic regimen to use.
Level of evidence: Moderate.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
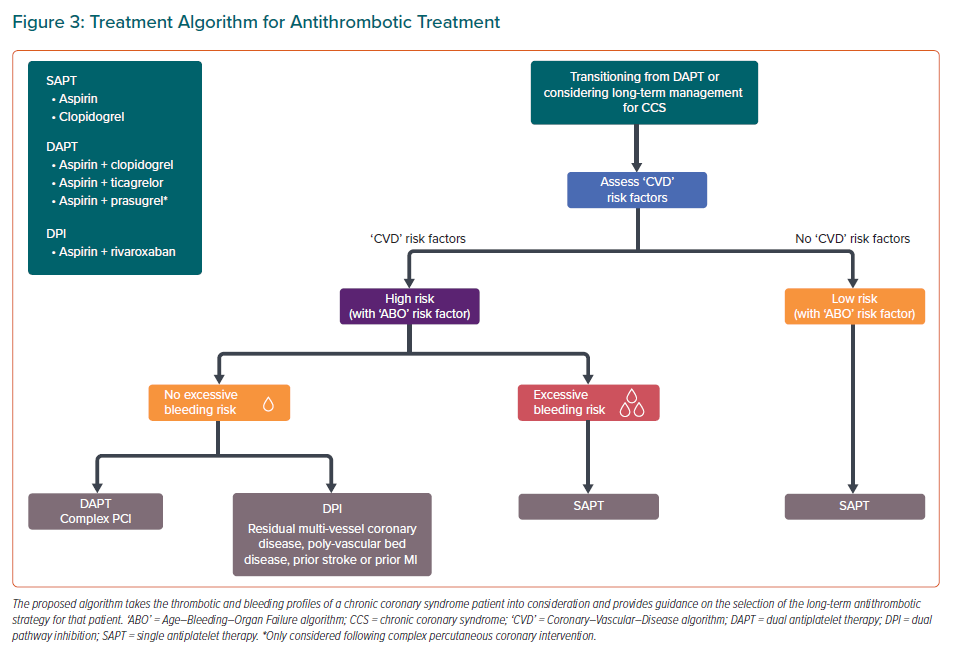
While minimising bleeding risk is important, so too is ensuring that antithrombotic treatment maximises therapeutic benefits of preventing thrombosis in CCS patients. In this consensus statement, we have developed two treatment algorithms, one for determining excessive bleeding risk and another for high thrombotic risk, to identify CCS patients who may require additional consideration when selecting an antithrombotic treatment strategy. Beyond improving the outcomes of CCS, personalising the intensity of antithrombotic treatment based on balancing thrombotic and bleeding risks of patients has shown to improve health utility and reduce the cost of CV events.15
Statement 6. Sex alone should not be considered when assessing bleeding and thrombotic risk.
Level of evidence: Very low.
Level of agreement: Agree 100%, neutral 0%, disagree 0%.
In the assessment of thrombotic and bleeding risk of a patient, female sex has been reported to confer both a higher thrombotic and bleeding risk, possibly due to the smaller body habitus of women.16–18 However, the association of thrombotic and bleeding risk with sex has not been confirmed definitively in Asian populations.18 The consensus panel felt that body habitus alone is insufficient to determine thrombotic and bleeding risk and recommended removing sex from consideration when assessing such risks.
Bleeding Risk Assessment
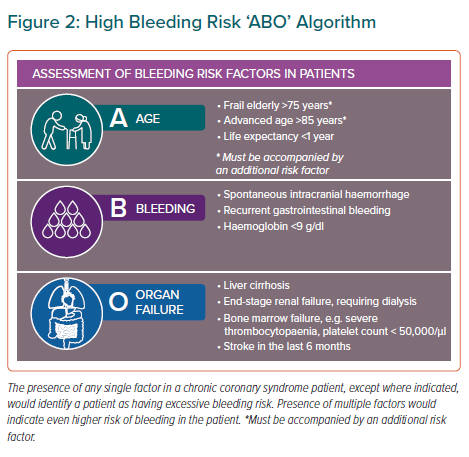
Statement 7. The Age–Bleeding–Organ Dysfunction (‘ABO’) algorithm can be used as a binary approach to excessive bleeding risk.
Level of evidence: Low.
Level of agreement: Agree 92.0%, neutral 8.0%, disagree 0%.
Statement 8. Advanced age alone is insufficient to confer excessive bleeding risk in CCS patients in Asia Pacific.
Level of evidence: Very low.
Level of agreement: Agree 76.0%, neutral 24.0%, disagree 0%.
Statement 9. Haemoglobin levels <9 g/dl (<5.6 mmol/l) should be used as an indication for anaemia in Asian patients when assessing bleeding risk.
Level of evidence: Very low.
Level of agreement: Agree 64%, neutral 36%, disagree 0%.
Various bleeding risk scores have been proposed for use in the clinic, including the Academic Research Consortium for High Bleeding Risk (ARC-HBR) and the HAS-BLED scores.19,20 These scores have not been validated in Asian populations and are not widely used in clinics in Asia. The consensus panel proposed the use of the ‘ABO’ algorithm as a simple approach to identify patients with excessive bleeding risk, based on ‘Age’, ‘Bleeding’ and ‘Organ dysfunction’ factors (Figure 2). This algorithm employs a binary approach where the presence of any single factor indicates excessive bleeding risk in a patient.
Age
Age is a common risk factor featured across most bleeding risk scores.19,20 Increasing age has been strongly associated with an increase in bleeding risk. In a cohort study of 3,166 patients, the risk of non-major bleeding was unrelated to age.21 On the other hand, major bleeding increased with age ≥75 years in patients with a first transient ischaemic attack, ischaemic stroke or MI treated with antiplatelet drugs (HR 3.10; 95% CI [2.27–4.24]; p<0.0001), particularly for fatal bleeds, and was sustained during long-term follow-up.21 As a result, even with higher risk of death and CV events, the elderly were consistently under-treated with antithrombotics. Such elderly patients could potentially benefit from antithrombotic treatment but miss out on therapeutic benefits due to the fear of major bleeding events.22
Advanced age is often compounded with other risk factors that similarly confer bleeding risk including prior stroke and gastrointestinal bleeding, hypertension, anaemia, renal insufficiency and presence of cerebrovascular disease.23 In addition, advanced age is associated with frailty, which is thought to be reflective of an individual’s biological age and deemed to be a more accurate predictor for the occurrence of bleeding events compared with chronological age.24 Coupling these with the ARC-HBR considering age as a minor criterion for bleeding risk, the consensus panel suggests that using an age cut-off of ≥75 years on its own would not be a strong enough indicator of excessive bleeding risk for CCS patients in the Asia-Pacific region. 25 Rather, CCS patients aged ≥75 years should be identified to have excessive bleeding risk only when accompanied by frailty and another independent risk factor, and for those of advanced age >85 years to be identified as having excessive bleeding risk when accompanied by another independent risk factor. As frailty scores and scales are not commonly used and have not been validated in the Asia-Pacific regions, the determination for frailty should be based on clinical judgement.
Bleeding
Prior serious bleeding events such as intracranial haemorrhage, recurrent gastrointestinal bleeding or anaemia with haemoglobin <9 g/dl (<5.6 mmol/l) were also highlighted by the consensus panel as key risk factors relevant in Asia for predicting bleeding.19,26,27 For anaemia, ethnic differences of mean haemoglobin levels have been reported, suggesting the need to reconsider ethnicity-specific cut-offs when identifying anaemia in Asia.28 With many on the consensus panel reporting the observation of lower mean haemoglobin levels in healthy patients in their clinics, a lower cut-off for haemoglobin levels to 9 g/dl was recommended as an indication of anaemia.
Organ Dysfunction
Beyond age and bleeding, organ dysfunction, including liver cirrhosis, end-stage renal failure and severe thrombocytopenia, and prior stroke in the last 6 months have also been implicated as major predictors of bleeding.19,27 With liver cirrhosis, most patients have coagulation abnormalities resulting in higher prothrombin time-derived international normalised ratios, which increases bleeding risk in these patients. Coagulation abnormalities as a result of reduced platelet counts from bone marrow dysfunction also increases bleeding risk. Severe thrombocytopenia as defined by platelet count <50,000/µL has been shown to increase bleeding risk (OR 3.1; 95% CI [2.0–4.8]).29 Chronic kidney disease (CKD) is also associated with increased risk of bleeding. Patients with moderate-to-severe CKD were found to have the highest bleeding risk at a 3.5-fold increase in bleeding risk compared with patients without CKD.30,31
Thrombotic Risk Assessment
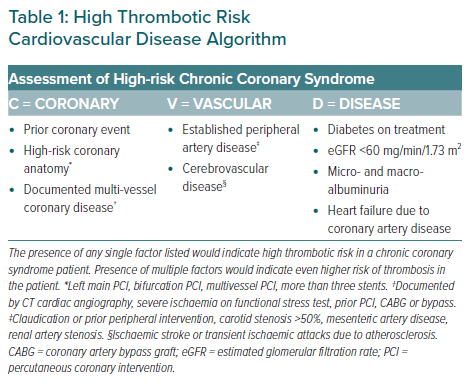
Statement 10. The Coronary–Vascular–Disease (‘CVD’) algorithm can be used to determine if a CCS patient has high thrombotic risk.
Level of evidence: Low.
Level of agreement: Agree 92.0%, neutral 8.0%, disagree 0%.
Employing a similar binary approach to identify CCS patients at high risk of thrombosis, the ‘CVD’ algorithm (Table 1) was developed to summarise individual patient risk factors that would confer high thrombotic risk to provide guidance on identifying such patients for antithrombotic treatment (Table 1). The risk factors for thrombosis included in the ‘CVD’ algorithm have been classified by ‘Coronary’ factors, ‘Vascular’ factors and ‘Disease’ factors.
Coronary
The ‘coronary’ factors identified to confer high thrombotic risk CCS patients include prior coronary events, documented multi-vessel coronary disease and high-risk coronary anatomy. Prior coronary events, such as MI and/or revascularisation, have been found to increase risk of thrombotic events (HR 1.44; 95% CI [1.40–1.49]), as well as rates of ischaemic events from 4.7% in year 1 to 15.1% in year 4 post-event.32,33
Multi-vessel coronary disease, as documented by CT cardiac angiography, severe ischaemia on functional stress test, prior PCI or coronary artery bypass grafting, has also been shown to increase risk of CV events (HR 4.18; 95% CI [3.66–4.77]) and reduce overall survival by up to fourfold.34,35 Complex coronary anatomical features, such as left main coronary artery stenosis and multi-vessel disease, and procedural factors, such as bifurcation PCI, have been identified by the clinical SYNTAX score as contributors to thrombotic risk.36 These factors have been included in the proposed ‘CVD’ algorithm (Table 1), where presence of any one of these factors would indicate a patient for high thrombotic risk.
Vascular
Patients with established peripheral artery disease (PAD), as defined by claudication, prior peripheral intervention, carotid stenosis >50%, mesenteric artery disease or renal artery stenosis, had higher rates of adverse CV events compared with patients without disease (5.35% versus 2.15%).5 In patients with CCS, concomitant disease in the vasculature, which includes both PAD and cerebrovascular disease, further increased the risk of thrombosis (HR 1.99; 95% CI [1.78–2.24]).37
Disease
Comorbid conditions included in the ‘CVD’ algorithm that indicate high thrombotic risk in CCS patients include type 2 diabetes with treatment, impaired renal function (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73m2), any degree of micro- and macro-albuminuria and heart failure as a result of CCS. Patients with CCS and type 2 diabetes have a two- to fourfold increase in mortality risk, with CAD being the main cause of death in these patients.38 Renal dysfunction has also been implicated as a risk factor indicating high thrombotic risk. As eGFR decreases below 60 ml/min/1.73m2, mortality risk increases –compared with an eGFR of 95 ml/min/1.73m2, patients with eGFR of 60 ml/min/1.73m2 have an HR of 1.03 while those with an eGFR level of 15 ml/min/1.73m2 have an HR of 3.11.39
In addition, micro- and macro-albuminuria at any level has been reported as an independent predictor of all-cause mortality in CCS patients,39 with HR 2.08 (95% CI [1.30–3.32]) for low-to-medium microalbuminuria and HR 1.99 (95% CI [1.08–3.70]) from high microalbuminuria to macroalbuminuria.40 Finally, patients with heart failure due to CCS have been documented to have poorer prognoses.41 The SOLVD study reported a fourfold increase in mortality risk in patients with CCS and heart failure.42
Selection of Antithrombotic Therapy for Asian Chronic Coronary Syndrome Patients
Statement 11. Single antiplatelet therapy (SAPT; aspirin or clopidogrel), rather than DAPT, is recommended for Asian CCS patients with low ischaemic risk or excessive bleeding risk.
Level of evidence: High.
Level of agreement: Agree 96.0%, neutral 4.0%, disagree 0%.
Statement 12. Extended DAPT is recommended for Asian CCS patients without high bleeding risk features, and who have also undergone PCI with complex stent features.
Level of evidence: High.
Level of agreement: Agree 92.0%, neutral 8.0%, disagree 0%.
Statement 13. The Coronary–Vascular–Disease (‘CVD’) algorithm can be used to determine ifDual pathway inhibition therapy (aspirin + rivaroxaban) is recommended for Asian CCS patients with high thrombotic risk and without high bleeding risk, and who also have residual multi-vessel coronary disease, poly-vascular bed disease, prior stroke or prior MI.
Level of evidence: Moderate.
Level of agreement: Agree 92.0%, neutral 8.0%, disagree 0%.
Following assessment of thrombotic and bleeding risk of CCS patients, clinicians are faced with the task of selecting the optimal antithrombotic therapy for their patients. The consensus panel developed a practical algorithm to guide antithrombotic selection for long-term management of CCS patients (Figure 3). This algorithm considers different thrombotic and bleeding risk profiles of CCS patients, as assessed using the ‘ABO’ and ‘CVD’ algorithms, to determine the optimal antithrombotic strategy for each patient profile type.
Low Thrombotic Risk or Excessive Bleeding Risk
The ESC guidelines for CCS recommend for SAPT to be considered in patients without a history of MI or revascularisation, but with evidence of CCS.1 Aspirin may be used for the long-term antithrombotic management of CCS patients with low thrombotic risk or excessive bleeding risk to reduce the risk of future CV events.43 When aspirin is unsuitable, long-term administration of clopidogrel to CCS patients has been shown to have a slight benefit over aspirin in reducing thrombotic risk, with similar bleeding rates to that of aspirin.44
The STOPDAPT-2 and SMART-CHOICE randomised clinical trials investigated the shortening of the DAPT duration from 12 months to 1 or 3 months, respectively, following PCI with the use of clopidogrel monotherapy thereafter. These trials reported potential benefits of switching to SAPT sooner following PCI, with lower or similar rates of CV events (HR 0.64; 95% CI [0.42–0.98]; p=0.04 for superiority in STOPDAPT-2 and 2.9% [SAPT] versus 2.5% [DAPT]; 1-sided 95% CI, [−∞, 1.3%]; p=0.007 for noninferiority in SMART-CHOICE) compared with 12-month DAPT duration. Significantly lower rates of bleeding events (HR 0.26; 95% CI [0.11–0.64]; p=0.004 for superiority in STOPDAPT-2 and HR 0.58; 95% CI [0.36–0.92]; p=0.02 in SMART-CHOICE) compared with 12-month DAPT duration were also reported.45,46 This suggests the potential utility of clopidogrel monotherapy for use in Asian CCS patients with excessive bleeding risk.
For CCS patients with low thrombotic risk or excessive bleeding risk, who also have a separate indication for oral anticoagulation such as AF, non-vitamin K oral anticoagulants may be considered in place of SAPT.
High Thrombotic Risk and Without Excess Bleeding Risk
Multiple randomised controlled trials have compared the efficacy and safety of extended DAPT duration of >24 months with standard DAPT duration of 12 months. The DES LATE and OPTIDUAL trials investigated the use of aspirin with clopidogrel with an extended DAPT regimen following PCI and reported no differences in both CV outcomes and bleeding events between extended versus standard DAPT duration.47,48 Beyond one year post-PCI, the DAPT trial reported significantly reduced rates of major adverse cardiovascular and cerebrovascular events with aspirin and clopidogrel/prasugrel compared with aspirin alone (4.3% versus 5.9%; HR 0.71; 95% CI [0.59–0.85]; p<0.001) although this was associated with increased moderate or severe bleeding (2.5% versus 1.6%, p=0.001).49 Similarly, the PEGASUS-TIMI 54 trial reported significantly reduced risk of CV mortality, MI or stroke by up to 15% with extended DAPT duration compared with a 12-month DAPT duration, with increased risk of major bleeding (2.60% versus 1.06%; p<0.001).50 Although these trials have indicated mixed results, the use of an extended DAPT regimen as a long-term antithrombotic strategy for CCS patients may provide significant benefit that outweighs the risk of bleeding, especially for patients with high thrombotic risk and without excess bleeding risk.
In the COMPASS trial, use of rivaroxaban with or without aspirin was evaluated for use in high-risk CCS patients, including those with PAD, diabetes, heart failure, prior stroke/MI and impaired renal function.51 The COMPASS trial reported that CCS patients with high thrombotic risk and without excess bleeding risk, who received low-dose rivaroxaban plus aspirin had significantly better CV outcomes with 23% relative risk reduction of mortality; although major bleeding events increased.51,52 For patients with polyvascular territory disease (i.e. CCS and PAD), the use of rivaroxaban with aspirin may provide additional benefits of improved peripheral limb outcomes as observed in the VOYAGER PAD randomised clinical trial.53
Conclusions
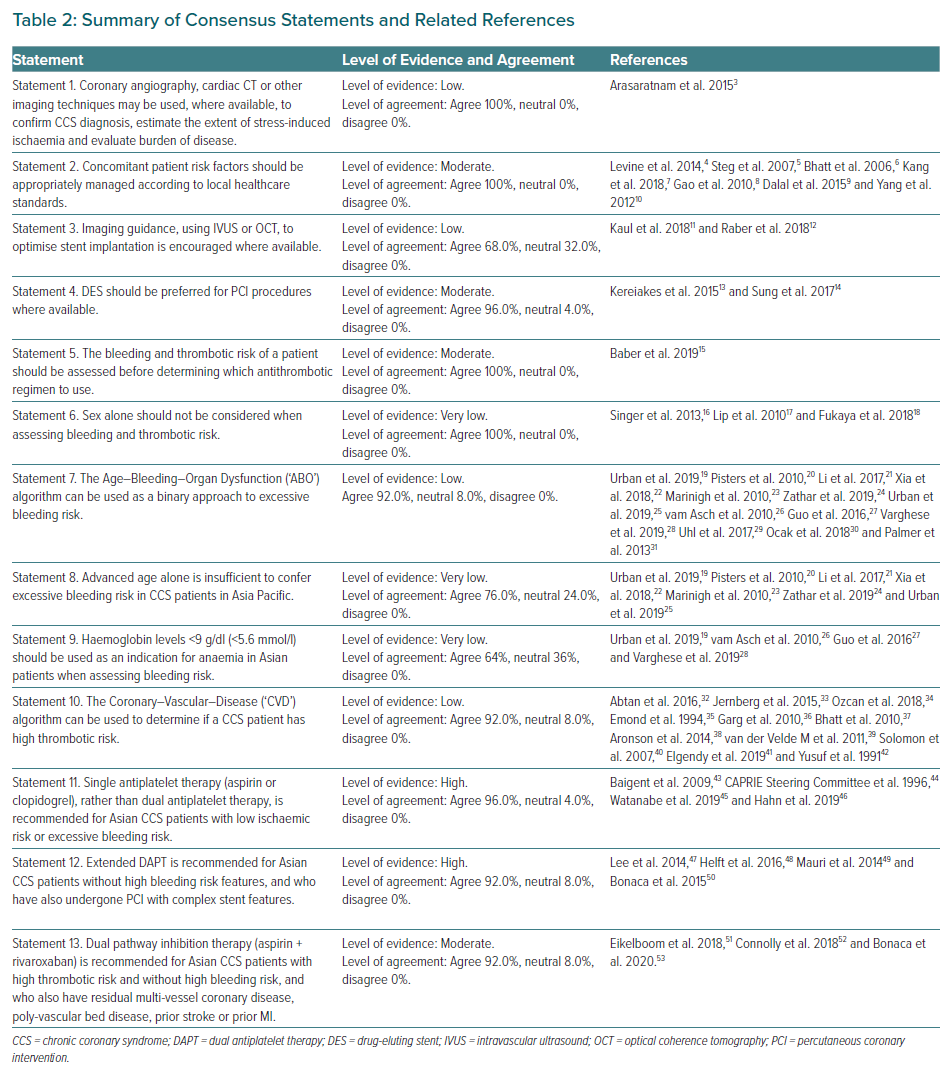
The consensus statements presented here, summarised in Table 2, aim to serve as a practical guide for clinical practice in the Asia-Pacific region to navigate the complex CCS landscape for antithrombotic management and improve CCS patient outcomes. The topics covered by the statements reflect the issues relevant to contemporary clinical practice in the Asia-Pacific region. The algorithms proposed aim to simplify and expedite the identification of CCS patients with excessive bleeding risk and/or high thrombotic risk in the clinic, as well as to guide selection of the optimal antithrombotic strategy and improve patient outcomes.