Globally, cardiovascular disease (CVD) accounts for 8.94 million deaths in women, comprising 35% of all deaths in women, making it their leading noncommunicable cause of mortality worldwide, with more women dying from CVD than from all cancers combined.1 In addition, by the last estimation, 275 million women are living with CVD.1
Despite this, women continue to be inadequately treated when they are diagnosed with CVD, have worse outcomes and have been historically excluded from clinical trials, especially around ischaemic heart disease (IHD).2,3 An approach to the unmet cardiovascular needs of women has been the establishment of Women’s Heart Centres (WHCs), with the ultimate goal of reducing disparities in cardiovascular care for women to improve outcomes.3–5 We aim to briefly review the need for these specialised centres and provide guidance for expanded development of WHCs globally to contribute to the achievement of this ultimate goal.
Need for Women’s Heart Programmes
The need for improved cardiovascular care for women was brought to light in the late 1990s, with the recognition that cardiovascular mortality in women had been steadily increasing for almost two decades while, during the same period, a notable decline had been observed in men. The Heart Truth and Go Red For Women public awareness campaigns were established in 2002 and 2004, respectively.6
More recently, an international commission from the Lancet emphasised the need for equitable cardiovascular care for women. It identified knowledge gaps that persist in cardiovascular disease that need to be addressed, and that this was central to reducing the global burden of cardiovascular disease in women.7
In response to these identified needs being highlighted, numerous private practices, hospitals and universities responded by establishing of a variety of centres and programmes dedicated to cardiovascular care for women.5
These centres, which deliver cardiovascular care focused on women, are known by various terms, depending on the institution in which they are located, including women’s heart centres, programs, or clinics. For the purpose of this review, we will refer collectively to these specialised practices as women’s heart centres (WHCs).
Although some may have early on discounted WHCs as a marketing tool, the need for improved cardiovascular care of women has only increased, with recent recognition that although, initially declining, CVD mortality has been rising again in both women and men, but disproportionately in younger and midlife women.8,9 Additionally, awareness in women about their risk for CVD as the leading cause of death has unfortunately decreased in recent years and this, combined with a surprising lack of training and preparedness in internists and specialists to assess and manage CVD risk in women, is alarming.10–11
As emerging research in women’s cardiovascular health is rapidly evolving, WHCs can provide expert consultative services to partner with other healthcare providers, providing contemporary sex-specific, patient-centred preventive and therapeutic care, while also collaborating on professional and public education strategies.
The first document to provide guidance towards developing WHCs was published by our group in 2016.5 The intention of this document was not just to provide expertise for CVD prevention and care but also to encourage the establishment of women-focused cardiovascular centres. The need for a team approach, collaboration with other medical specialties and consultative resources, was outlined in this document.
Following this document, a white paper from the American Heart Association expanded on the need and components of WHCs.12 It also established the need for WHCs as a new care model, emphasised the lack of and requirement for sex- and gender- specific education to all trainees, in addition to providing a rationale for expanding research in this area.
Gaps in Cardiovascular Care for Women
Inadequate Treatment
Women are often undertreated compared with men, as large registries of ST-elevated MI (STEMI) demonstrate. Women with STEMI are less likely to receive guideline-directed medical therapies, less likely to undergo revascularisation or receive thrombolytics, and are less likely to be referred to cardiac rehabilitation.13–16 Those with cardiogenic shock with STEMI obtain less aggressive care, including a lower likelihood of receiving mechanical circulatory support, and have a higher mortality rate.17 These all contribute to worse outcomes for women.
Similarly in AF, women are less likely to be offered a rhythm-controlled strategy, less likely to be offered catheter ablation and less likely to receive oral anticoagulation.18,19 Women with heart failure who meet criteria for ICDs, which have the potential to improve survival by preventing sudden death, are less likely to receive such devices than men.20,21
Gaps and sex-based inequities exist in the literature relating to primary and secondary cardiovascular disease prevention, with women less likely to be adequately treated.22
Delays in Care
Delays in acute cardiovascular care for women are multifactorial and lead to delayed door-to-balloon times.23 There may be patient factors, as reported in studies of STEMI patients, where it has been found that women are less likely to present within 2 hours of onset of symptoms, and have longer intervals than men.24
Additionally, there are healthcare system delays whereby the healthcare team may not recognise when women are presenting with acute coronary syndromes, because of gaps in knowledge and understanding of the differences in symptoms between the sexes; more ominously, there may be delays because of sexism, whether conscious or unconscious.3
Research and Knowledge Gaps
Women have, historically, been excluded from cardiovascular research.25 Although efforts have been made to increase the inclusion of women, recent estimates of clinical trials continue to show an underrepresentation of female participants in cardiovascular research, particularly in coronary artery disease and heart failure trials.26,27
Even basic science studies often fail to consider sex as a biologic variable by avoidance of female cell lines or a failure to include females in animal studies, or a lack of sex-based reporting of their findings.28 Additionally, many clinical trials that include both men and women fail to report sex-disaggregated findings, limiting our knowledge related to differences between the sexes.29
Why Sex and Gender Matter
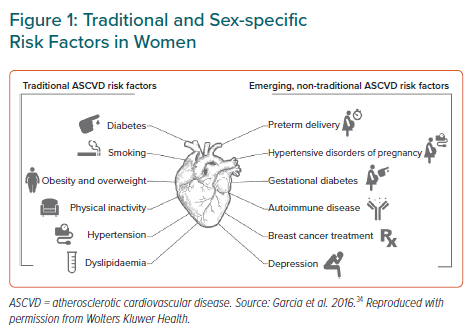
The term sex refers to the biological aspects of being male or female, while gender is a social construct influenced by an individual’s environment and includes gender identity.30 Both may impact upon cardiovascular health, including manifestations of traditional CVD risk factors, development of sex-specific risk factors, symptom recognition and diagnosis, and, ultimately, treatment with pharmacologic and/or interventional therapies as indicated (Figure 1).3,31–34
It should not be surprising that many aspects of CVD differ in women and men. Non-obstructive coronary artery disease is a more frequent cause of IHD in women and manifests as INOCA (ischaemia with no obstructive coronary artery disease) or MINOCA (MI with no obstructive coronary artery disease), and has been underappreciated until recently.35,36
Heart failure with preserved ejection fraction is twice as common in women, in contrast with heart failure with reduced ejection fraction, which is more often seen in men. Interestingly, although women have higher left ventricular ejection fractions at baseline, sex-neutral thresholds have been used to define heart failure, with such sex-specific differences in cardiac structure and physiology being disregarded completely.37
It is beyond the scope of this review to describe all sex differences in CVD; these foregoing examples are but a few that illustrate the need for WHCs that focus on sex differences in the prevention and diagnosis of CVD, in addition to the specialised treatment of CVD conditions unique to women.
Structure of Women’s Heart Centres
Foundational to most WHCs is a team-based approach to the cardiovascular care of women.5,38 This includes cardiologists and advanced practice professionals who may work together as the main providers. Additionally, collaboration with other specialists and services in medicine and surgery are often necessary, including cardiac rehabilitation, nutritional services, physical and occupational therapists, rheumatologists as well as genetics, vascular, neurologic, mental health, obstetrics-gynecologic, endocrinologic and integrative specialists.
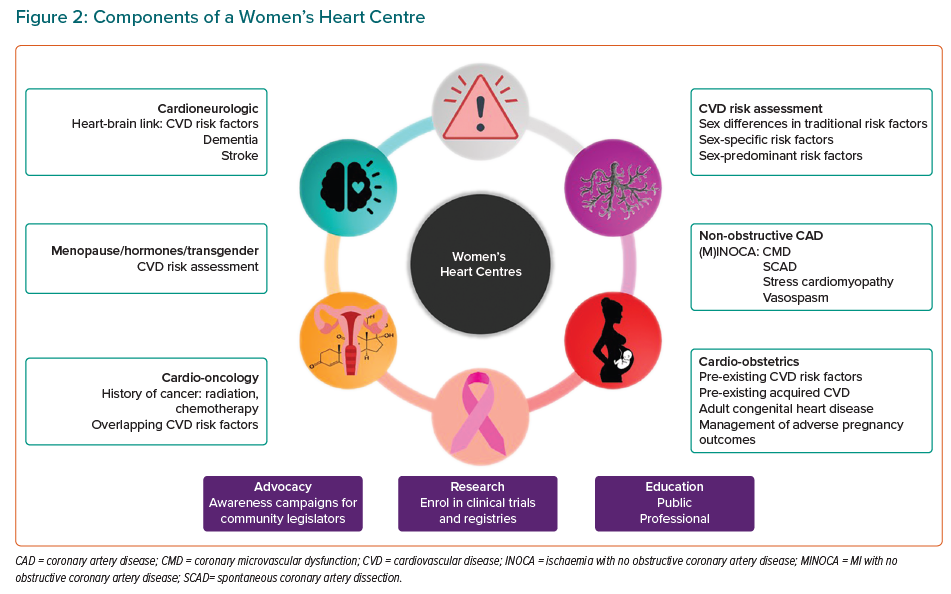
WHCs are not simply confined to outpatient care but are integral to the entire cardiovascular care of women, based on expertise and education that can be delivered in different aspects of care. Although it may not be possible for all WHCs to offer every component outlined in Figure 2, our intent is to present an approach to a comprehensive programme that addresses sex-specific issues in the cardiovascular care of women. A WHC may include the cardiovascular services below.
Cardiovascular Disease Risk Assessment
This may be available to any woman, with or without a prior cardiovascular disease history, using a combination of traditional, sex-specific and sex-predominant risk factors to provide short-term and life-time risk assessments.33 This can also include secondary CVD prevention, ensuring that guideline-directed medical therapies and cardiac rehabilitation are used to improve cardiovascular outcomes in women.
Non-obstructive Coronary Artery Disease
Women with ischaemia or MI, where there are no obstructive coronary arteries, often require further assessment to identify the cause. INOCA and MINOCA are not final diagnoses but classifications that include several potential diagnoses. Further diagnostic testing is frequently required to obtain an aetiologic explanation for the perplexing dilemma of ‘angina and/or MI with normal coronary arteries’. Medical management must be tailored according to the specific diagnosis, which may include coronary microvascular dysfunction (CMD), coronary vasospasm, spontaneous coronary artery dissection (SCAD) or stress (takotsubo) cardiomyopathy, in addition to other possible causes that can be elicited with a more extensive work-up.35,36
Cardio-obstetrics
There are numerous potential cardio-obstetric reasons for referral to a WHC. Every woman who is referred to a WHC should have a cardio-obstetrics history performed to uncover often unrecognised sex-specific cardiovascular risk factors. Women may be referred after an adverse pregnancy outcome (APO) and this may require cardiovascular risk assessment, medical management or, usually, both.
Additionally, women with familial hypercholesterolaemia, hypertension or other established cardiac conditions (both acquired or adult congenital heart disease) may be referred before conception or once the pregnancy is established. Partnering with maternal-foetal medicine creates an important link for women during this critical period.
Cardio-oncology
Although cardio-oncology clinics may be separate from a WHC, if there is not a specialised clinic, it is possible that women who have previously received certain cancer therapies (e.g. breast radiation therapy or systemic chemotherapy) may be identified to be at a higher risk for CVD.39 Risk factors for breast cancer, which occurs far more frequently in women, overlap with risk factors for cardiovascular disease. Cardiovascular risk assessment is important in these women and can be addressed in WHCs.
Cardio-rheumatology
In patients with autoimmune disorders, chronic inflammation increases CVD risk.40 Collaboration with rheumatologic specialists may be beneficial in women with these disorders for assessment and guidance on management of increased CVD risk, and/or optimisation of treatment if heart disease has become manifest.41
Menopause, Hormones and Cardiovascular Disease
Menopause can often be a time when women are referred as part of seeking care related to menopausal symptoms. Additionally, women may experience unfavourable changes in CVD risk with menopausal-related changes in cholesterol, elevations in blood pressure or increased weight gain and abdominal adiposity that result in referral to a WHC. Understanding the impact of hormones, not just on symptoms but also on potential cardiovascular risk, is an important requirement for providers participating in informed and shared decision-making with women who are in this phase of life.
Cardioneurologic/Geriatric
The awareness of the heart–brain connection is increasing. As risk factors for CVD overlap with those for stroke and vascular cognitive impairment leading to dementia, collaboration with neurology, geriatrics and vascular medicine can be an important aspect of a WHC.
Transgender Cardiovascular Health
This is an emerging area of importance, given the increased cardiovascular risks that have been reported.42 Hormonal changes, and their impact on cardiovascular risk need to be further studied, but risk assessment and management could fall within the domain of a WHC.
Other factors that must be considered in the establishment of a WHC include accessibility, cultural and ethnically appropriate care and educational material, both public and professional.12 Additionally, when a WHC is established within an academic medical centre, it is important that the educational component includes the education of medical students, residents and cardiology fellows. Incorporation into the academic curriculum will help broaden the knowledge of appropriate care of women, and further the understanding of the sex differences in cardiovascular disease.
WHCs can also incorporate research into their mission. Where possible, establishment of a database registry to follow patients and report on outcomes data could advance the field further. WHCs in academic and clinical research settings may also include opportunities for recruitment into clinical trials to assist in increasing the enrolment of women and expand a knowledge base needed for evidence-based guidelines development.
Growth of Women’s Heart Programmes
There is no regulatory requirement to register WHCs so it is difficult to ascertain how many exist on a global scale. However, there are now WHCs in Australia, Asia, Europe and the Middle East, with the largest numbers are in North America. Each WHC is unique in terms of its funding and clinical programme components; some may deliver exclusively outpatient care. With any WHC, there are opportunities for the entire delivery of cardiovascular care to be transformed; an understanding of sex differences begins with the recognition of differences between men and women and may begin with education on such differences among other providers. Considering the vast population of women at risk at and living with CVD, the need for specialised care for women could potentially be better served with further global expansion of WHCs.
Certainly, the need for WHCs is global, but there are worldwide differences in healthcare that can create barriers to establishing them and affect reimbursement for care.7 As there are knowledge gaps and awareness of the unique aspects of cardiovascular care for women is evolving, expertise in this care has been centred on WHCs, which are usually located within a cardiology division or department. Therefore, patients seen in WHCs should have the same access and coverage as any patient seen by a specialised cardiovascular care team.
Additionally, the creation of WHCs can allow women access to clinical research trials within university and hospital systems, and enrolment into registries from any centres. This should be part of development of any WHC (Figure 2).
There are a few unaccredited training programmes for women’s cardiovascular health but no established certified training in this specialty, creating a limitation in expertise in the care of women.
Additionally, there is no evidence to date demonstrating a direct improvement in the care of women or improvement in cardiovascular outcomes related to the establishment of WHCs. Indeed, because of the complexity of cardiovascular care issues and global population heterogeneity, this may never be able to be demonstrated as a direct effect, and these data may eventually need to be indirectly inferred.
Nonetheless, the persistent gaps seen globally require an urgent change in the established, traditional ways of delivering cardiovascular care to women, who comprise the majority of the population. WHCs have been set up to address this urgent need.
Conclusion
Specialised WHCs provide a unique, long-term, sustainable solution to address ongoing deficiencies in cardiovascular health provision for women. The dedicated, multidisciplinary and multispecialty teams in WHCs provide the best opportunity to deliver high-quality research and individualised, accessible care with appropriate female-specific risk stratification and treatment.
Large-scale expansion of the female-focused approach to prevention and treatment delivered by WHCs may be the key to delivering what has been lacking to date: a sustained, progressive decline in cardiovascular morbidity and mortality, saving the lives of women for years to come.