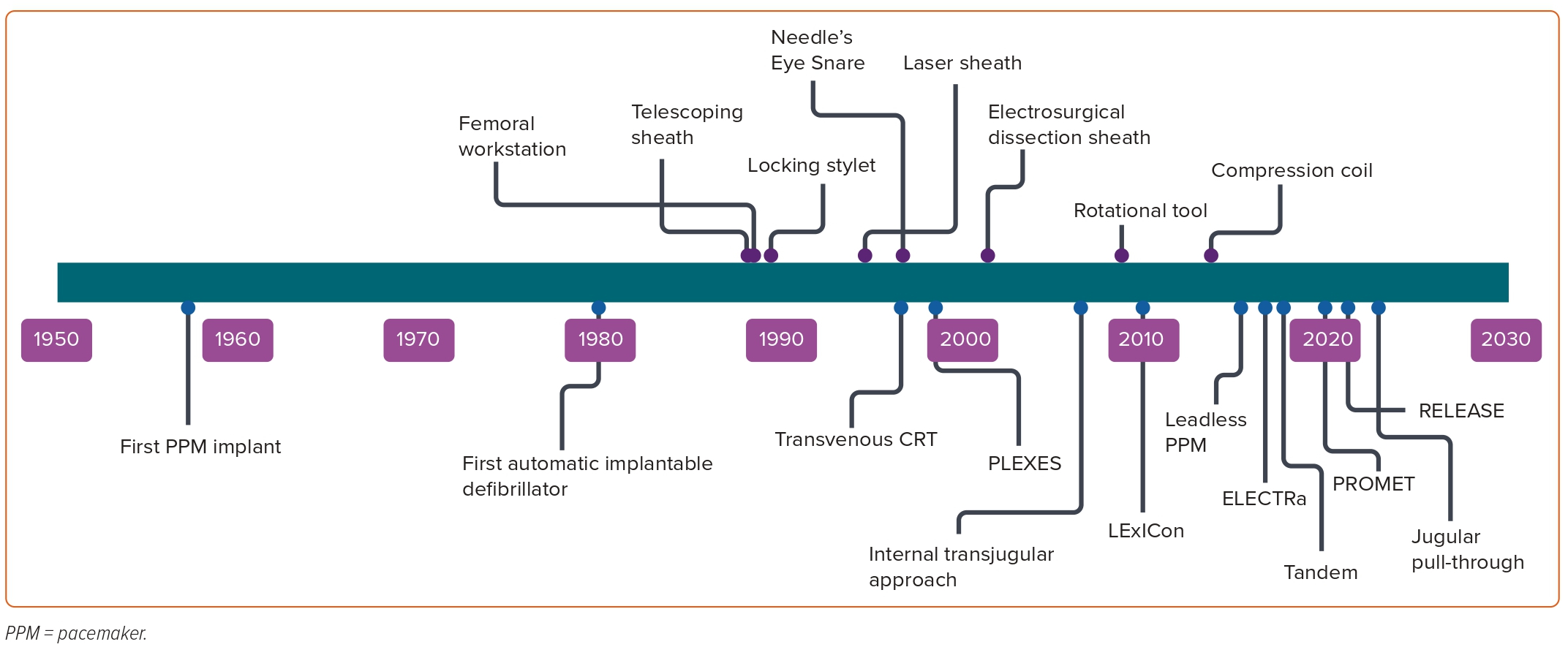
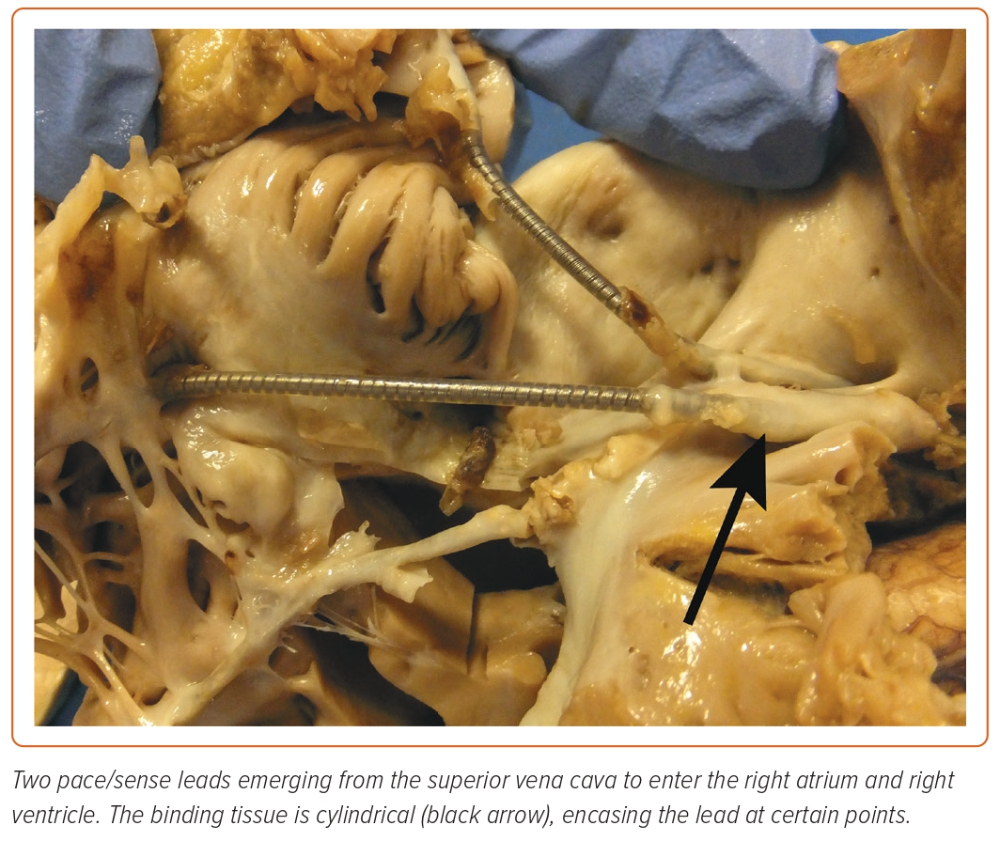
Cardiac implantable electronic devices (CIEDs) are central to the management of arrhythmias and to mitigating the risk of arrhythmia. In the UK alone, >40,000 devices are implanted each year.1 The extraction of leads is essential to the long-term management of CIEDs, establishing transvenous lead extraction (TLE) as a life-saving procedure. TLE has undergone an incredible evolution since its inception (Figure 1). Early methodologies relied on traction alone, applied from the original site of venous access; when this failed, cardiac surgery was used to complete the task.2,3
The traction used to extract leads was initially applied to the lead alone, but later enhanced by the use of locking stylets and by the use of snares to permit traction from femoral or jugular access points.4 For more challenging cases, traction-only methods were soon supplanted by methods combining dissecting sheaths with countertraction. The dissecting sheaths used in this process were initially simple tubes, sometimes used in a telescoping arrangement. The perceived technical difficulty of dissecting with these sheaths provoked the development of powered sheaths, in which radiofrequency or laser energy permits the advancement of the sheath with less physical effort. More recently, rotational sheaths have been used to achieve the same effect.
In parallel with the development of new extraction tools, the professionals performing TLE have developed protocols for maintaining safety during procedures and for preparing operators and institutions to perform TLE. Improved training has expanded the number of competent operators. Despite these advances, the risk of complication remains, including patient death, often due to injury to the superior vena cava (SVC).5 In this review, we aim to describe the principles of transvenous lead extraction, examine the challenges facing operators and evaluate current techniques.
Indications
The indications for TLE have evolved in response to improvements in procedure safety and efficacy. The most consistent indication for extraction is infection.6–8 Bacteria are able to colonise all surfaces of the non-biological material, including crevices, making infection near-impossible to eradicate completely from the surface once established.9 This is partly because colonising bacteria produce a glycocalyx biofilm, which permits the organisms to adhere firmly to the non-biological medium, and resist decolonisation from washout, antibiotics or bacteriophages.9 It is axiomatic that removal of all hardware is necessary to resolve device-related infection.10,11 Without removal, the outcome is generally poor; in a retrospective study of 416 patients with CIED infections, 30-day mortality was sevenfold higher in the group treated with antibiotics alone, while infection has been associated with an elevated risk of 1-year mortality in a cohort of patients, even after TLE.12,13 The guidelines have unsurprisingly mandated hardware removal following an infection, local or systemic.14
The venous system is prone to occlusion following introduction of leads, a process caused by thrombus, fibrosis or both. The prevalence of occlusion ranges between 2% and 22%, possibly higher in those with infection.15 In a small minority of patients, the effects of venous occlusion can be debilitating, necessitating extraction of the leads to facilitate correction of the occlusion.8 Most patients with venous occlusion remain asymptomatic, and no intervention is required as long as the CIED system functions well. If a lead has to be replaced or added to permit continued or enhanced CIED therapy, extraction of one or more leads can provide access for the new lead(s).
The use of TLE in managing malfunctioning or redundant leads requires careful consideration, as extraction is not mandatory; abandonment rather than extraction may be chosen. Current guidance stipulates a risk versus benefit approach on an individual basis for either strategy. As an approach, this is irreproachable, but the published literature does not provide an adequate basis for the calculations required to apply this guidance objectively. The risk of death associated with extraction is lower in the recent literature than previously (1–2%).5,16 The potential for harm from abandoned leads is difficult to quantify, as the risk can stretch over decades. It includes the risk of infection, thrombosis and the higher complication risk associated with deferred TLE (if required).
The dilemma of abandonment versus extraction has not been addressed by any large, randomised trial. A registry study of 6,859 patients comparing the outcomes of abandonment and extraction found that abandoning the leads was associated with a higher risk of infection than TLE, necessitating lead extraction at a later date.17 The complexity of the subsequent procedure is enhanced by the initial deferral. A study of 1,386 patients undergoing TLE for infection compared the outcomes in patients with and without previously abandoned leads. The TLE procedures took longer in patients with abandoned leads, requiring more bail-out approaches, with a higher rate of incomplete success and complications.18 Vegetations were also more prevalent with abandoned leads and, when present, were larger than in patients without any abandoned leads, probably due to the greater surface area created by the extra hardware within the vasculature. The outcome of TLE was poorer in patients with previously abandoned leads, not only in metrics of efficiency but also in 30-day mortality.18
Methods
Traction and Countertraction
The first extraction method applied was direct traction of the targeted lead, which carried risk of cardiovascular injury. There has been significant progression from the use of traction alone to the combined use of traction, and countertraction exerted with a dissecting sheath. Traction alone is still used to remove leads that have been in place for only a short time, usually <2 years. In the PROMET trial, these accounted for approximately 17.8% of the leads extracted.5 Commonly, traction is exerted with the aid of a locking stylet to permit the transmission of force to the lead tip and minimise the risk of lead breakage; in the PROMET study, 24.4% of leads were extracted using this method.
With traction, the lead is transformed into a railroad for the dissecting sheath; countertraction is applied to advance the sheath over this railroad to dissect the lead free. Early dissecting sheaths consisted of polypropylene, Teflon or steel, each with distinct physical properties. Teflon, being soft, allowed the sheath to be flexible, albeit with compromised dissecting ability. The polypropylene sheath was stiffer, useful in dissecting the leads from challenging adhesions, but with a reduced ability to negotiate curves. A steel sheath was used only to dissect dense scars at the entry site to the central vasculature.
In the early years, the femoral approach was regularly used to complete extractions. Devices, such as the Dotter snare and a tip-deflecting wire, were used for targeted leads via a large sheath positioned in the right atrium (RA).2 In combination, these tools permitted grasping of the lead, and traction could be applied inferiorly to complete the extraction.2 These techniques were effective and safe, and provided an alternative to surgical extraction, which was the predominant method in the 1970s and 1980s.3
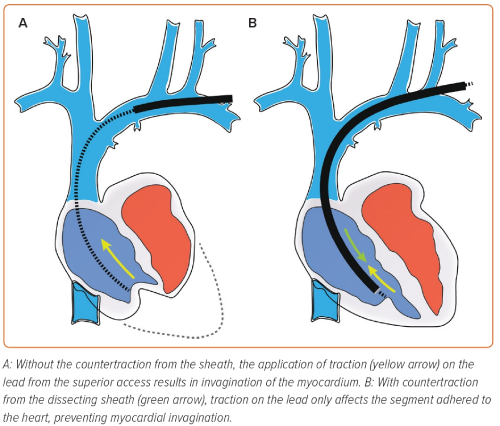
Although there have been advancements in the equipment used for lead extraction, the balancing of traction and countertraction remains central to all methods in TLE. Achieving equilibrium between the forces is essential – traction that is too forceful can cause avulsion, and forward force on the sheath without equal traction can elicit misguided dissection. Balancing the forces permits the operator to carefully advance the dissecting tool towards the lead tip, progressing steadily while dissecting the lead free of encapsulating tissue without causing injury or lead damage. The lead is withdrawn only when the sheath tip is in a position to provide counterpressure to the myocardium surrounding the lead tip (Figure 2).
Laser and Radiofrequency
Heat can be delivered to the sheath tip in the form of either laser or radiofrequency energy. These methods appear to improve the efficacy of extraction compared with simple sheaths. The theoretical advantage is the lower amount of force needed to dissect the lead free. The first powered sheath was the laser tool, which was conceived in the mid-1990s, adapted from percutaneous coronary intervention. Laser technology employs pulsed ultraviolet light with a wavelength of 308 nm at a repetition rate of 40–80 Hz to ‘vaporise’ encapsulating tissue. On a cellular level, this laser energy is absorbed by lipids and proteins, resulting in interruption of vital cellular bonds and causing tissue disintegration.19,20
Early evidence demonstrated laser to be more effective than the historical methods. The PLEXES study, a randomised trial consisting of 301 patients, compared laser lead extraction with the traditional non-laser methodology. The laser sheath achieved a significantly higher rate of complete extraction than the non-laser technique (94% versus 64%; p=0.001) and with a shorter procedure duration (10.1 ± 11.5 versus 12.9 ± 19.2 minutes; p<0.04).20 Laser lead extraction was considered an improvement on the traditional non-laser methods and remains popular.
However, there were significant methodological limitations in this study. PLEXES permitted cross-over, which may have introduced bias, and completing an extraction using polymer sheaths, but initiated with laser, was accepted as a laser lead extraction.20 Also, due to the low mortality rate in TLE (<2%), a randomised trial requires significantly more participants than the 301 patients of the PLEXES trial to unearth statistical significance for an adverse event endpoint.5,16,21 The LExICon study was a large observational study consisting of 1,449 patients and 2,405 targeted leads, evaluating the outcomes of laser lead extraction. It concluded that laser was safe and efficacious, achieving a high complete lead extraction (96.5%), with a major complication rate of 1.4% and mortality of 1.86%, figures that were considered satisfactory at the time.21 LExICon did have important advantages over PLEXES. The large population size permitted valuable statistical interpretation of the dataset, reducing the probability of ‘chance’ influencing outcomes. The retrospective nature of the study could be viewed as a limitation with confounding variables; however, it represented real-world experience. LExICon also better represented the safety and efficacy of laser lead extraction, as operators were more experienced with the technology and past the learning curve; PLEXES was better representative of the learning curve associated with laser-assisted extraction.
Electrosurgical dissecting sheaths apply radiofrequency energy to dissect the leads free from the encapsulating tissue, in a similar manner to cauterisation. The delivery of energy is local, with two poles situated at the distal tip of the extraction sheath; the application of heat is consequently linear between these poles, whereas application of energy by laser is circumferential. In comparison with the conventional dilatation technique, electrosurgical dissecting sheath tools are effective. A randomised trial of 120 patients compared electrosurgical dissecting sheaths with the conventional telescoping sheaths, and found that the powered sheath achieved a higher complete lead extraction (93% versus 73%; p<0.001, respectively).22 The overall complication rate was low in the whole study, leading to the authors concluding that TLE was relatively safe. Radiofrequency sheaths gained only a limited market share and were discontinued, being replaced by the rotational tool.
Rotational Sheath
The other category of powered sheath in current practice is the rotational tool. This motion-powered device features a stainless steel dissecting tip, which rotates with every hand-triggered activation. Like the laser sheath or simple mechanical sheaths, the rotational tool is fed over the lead, and with a balance of forward pressure and counter-traction, it is advanced as it dissects the lead free. The Evolution sheath (Cook Medical), with a unidirectional rotating mechanism, was superseded by the Evolution RL, with bidirectional rotation, as the first-generation device was prone to causing lead wrapping as it rotated in a single direction.23,24
Early experience of the rotational tool found it to be effective and safe, but these studies were small due to the relative novelty of the technique. PROMET is the largest study to date evaluating rotational tool TLE.23,24 This large observational study consisting of 2,205 patients with 3,849 targeted leads found that rotational tools were highly effective, with a complete success rate of 96.5% (clinical success 97%), and safe, with a major complication rate of 1%, including procedural mortality of 0.4%; there were no SVC injuries.5 The most significant finding was the high rate of success in leads of a significant dwell time (106 months; interquartile range 66–145 months), highlighting the strength of the rotational tool in disrupting dense, calcified scar tissue.5 The findings of PROMET were validated by the RELEASE study, a multicentre prospective trial with independent adjudication of complications. In this trial of 230 patients and 460 leads, clinical success (98.7%) and complete success per lead (96.3%) were similar to PROMET, with a major complication rate of 5.3% and procedural mortality of 0%.25
The TightRail (Philips) is a more recent rotational sheath. Although similar in appearance to the Evolution sheath, there are differences in stiffness and in tip design. Small series have revealed an effective and safe tool reaching complete success rates of 95–98%, and with a comparable safety profile to the Evolution sheath.26,27
Femoral and Jugular Extraction
In a small proportion of cases (<5%), lead extraction from the conventional subclavian approach proves unsuccessful.5,16,28 Failure in these cases is likely multifactorial. Lead dwell time is an established variate associated with failure of the conventional approach.28,29 Leads of a prolonged dwell time are encased by mature scar tissue often resistant to dissection, increasing the risk of procedural failure. These leads themselves are also of an older age, with sustained exposure to the shear forces within the vasculature. This can reduce their durability, provoking lead fracture during TLE; without a firm rail, the extraction sheath is ineffectual. The superior access also presents geometric challenges.30 There are significant angulations to navigate for dissecting tools during TLE from the subclavian access, which can reduce the tool efficacy. Severe angulations can also generate stresses on the lead, potentially compromising lead integrity and role as a rail for the dissecting sheaths.
Femoral access provides a versatile approach for the completion of percutaneous cardiac procedures and has traditionally been used as a ‘bail-out’ strategy during TLE.31 It offers important advantages over the subclavian approach (implantation vein). The femoral vein provides large, linear, direct access to the heart via the inferior vena cava and, therefore, has been used to overcome the challenging angulations associated with the subclavian access.32 The large bore access permits the use of sizable snares safely, and the direct pathway to the heart chambers provides a geometric advantage during application of extraction forces. In a small observational study, the use of femoral snare lead extraction was found to be a successful bail-out strategy; the overall rate of clinical success and complete lead extraction was improved with the addition of femoral snare extraction after the subclavian approach had failed; without this strategy, the procedural success would have been lower than contemporary expectations.31 In this study, the benefit of the inferior approach is highlighted by the removal of inaccessible leads, which were sited completely in the vasculature and challenging leads of a prolonged dwell time with passive fixation.
The importance of the inferior approach was validated by a large observational study comprising 1,080 patients. El-Chami et al. demonstrated that the inferior approach was required in a small percentage of cases, which comprised challenging cases: leads of a prolonged dwell time, numerous targeted leads and infection indication. Despite the challenges, the bail-out strategy was safe and effective in experienced hands.28
The advantages of the inferior approach can be maximised as a primary extraction strategy. Bracke et al. reported in their experience of 229 cases that femoral extraction with the use of the Needle’s Eye Snare (NES; Cook Medical) was effective and safe; 98.2% clinical success was achieved, with 0.7% major complications and 0% procedural mortality. This was safe and effective due to the linear path to the heart via the inferior vena cava, which provided a geometric advantage to extracting atrial and coronary sinus leads while reducing the risk of SVC injury. This is demonstrated by the <1% failure of atrial and coronary sinus lead extraction compared with 2.7% of right ventricle (RV) leads; RV lead removal requires the sheath to adopt an acute angulation as it enters the ventricle.33 This technique requires extensive experience to achieve a high efficacy and safety, shock leads are more challenging due to their size, and the application of force to free the lead from scar may result in lead damage, risking procedural failure.33
The jugular approach has advantages over the femoral: a linear path to the heart over a short distance. This enables the use of dissecting sheaths to complete the extraction, which is not possible with the inferior approach.34 Also, the jugular approach permits straightening of the targeted lead, which provides a straight rail for the extraction tool. This maintains the linear shape of the sheath, maximising its dissecting ability. Bongiorni et al. devised a unique technique to capitalise on the advantages of the jugular approach, the internal transjugular approach. This technique requires a multi-access approach (subclavian, jugular, femoral) to ‘pull down’ the targeted lead into the RA, rendering it free floating, and then ‘pull up’ out of the jugular to railroad a telescoping sheath for the extraction. In 213 targeted leads, the technique was highly effective, reaching a complete success rate (96.2%), comparable with powered sheath extraction, with a <1% major complication and mortality rate.35 Unlike the femoral snare extraction, the jugular technique is just as effective with large shock leads as with smaller pacing leads, and minimises the requirement to dissect in the SVC, as the lead is pulled through rather than dissected from some of the adhesions there, reducing the risk of SVC injury.35
Although highly effective and safe, the transjugular approach technique has limitations: as the targeted lead is pulled into the RA to render it ‘free-floating’, a locking stylet cannot be deployed, as it risks injuring the vasculature. Consequently, a powered sheath cannot be used in the usual manner. The lead has to ‘slide’ through the encapsulating adhesions for it to become ‘free-floating’, which may not always be possible; leads of a long dwell time may be bound by calcified encapsulation that requires powered sheath dissection. A novel technique has been described to overcome these limitations – transfer of a lead with a fully deployed locking stylet from a subclavian to jugular access point.34
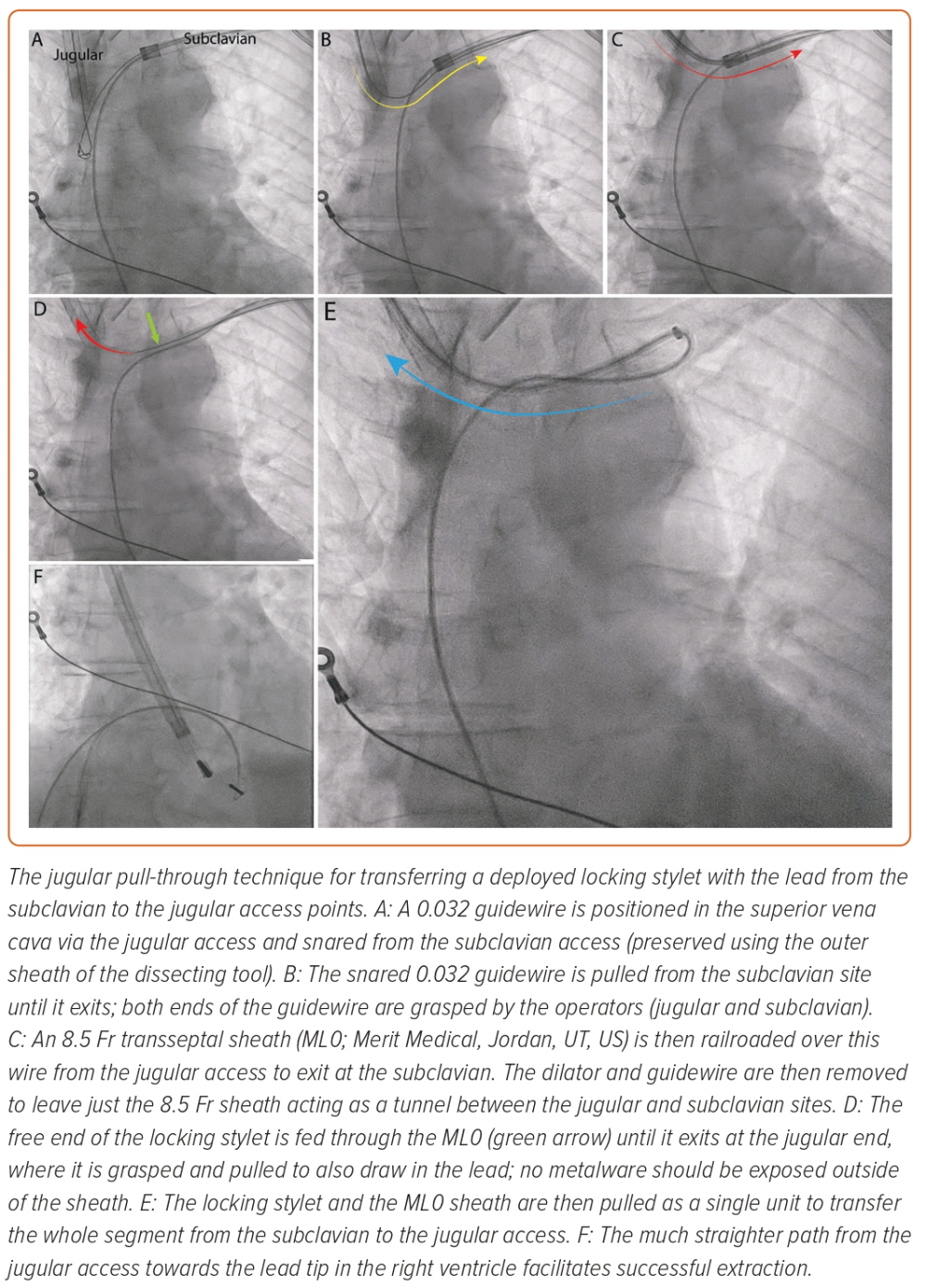
The Akhtar and Gallagher jugular pull-through technique has been demonstrated to be a feasible means of performing this transfer for completion of a challenging case (Figure 3).36 With this technique, the extraction can be started as normal, with the deployment of a locking stylet and advancement of a powered sheath. When needed, the unit can be transferred safely and reasonably quickly to the jugular for the powered sheath to exploit the linear direct path towards the lead tip. This technique uses a long sheath to act as a ‘tunnel’ for the metalware between the subclavian and jugular access points. Through this, the deployed locking stylet and lead unit can be transferred without exposure to the vasculature. The limitation of this method is that it requires two operators experienced in extraction. It may also add time to the procedure, which can be offset by jugular preparation from the beginning and switching to this approach early in the procedure.36
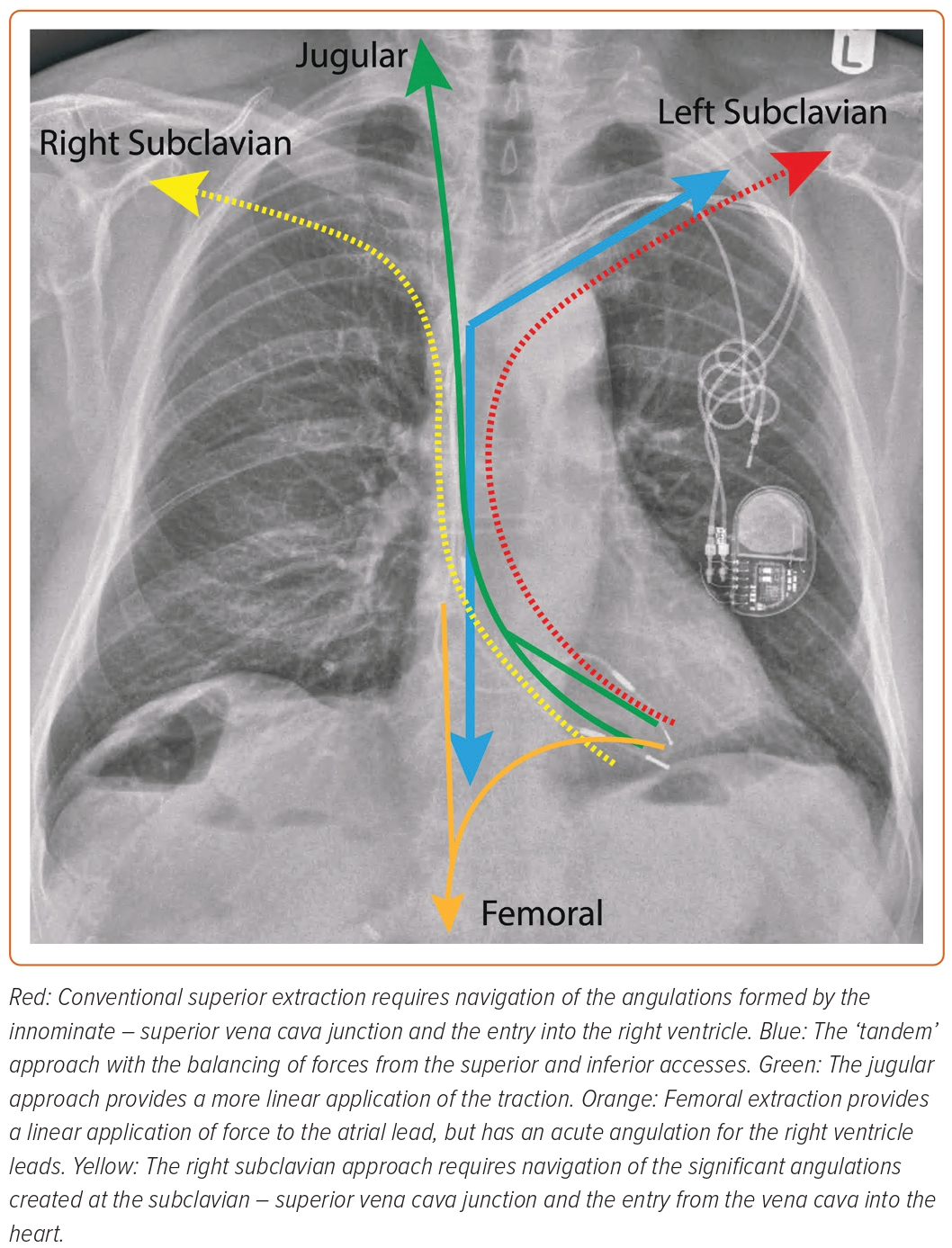
‘Tandem’ Approach
The control of extraction forces is crucial to a successful TLE, whichever equipment or route of access is used. The ‘tandem’ technique uses combined superior and inferior approaches to balance the applied forces (Figure 4).37,38 The targeted lead is held taut by the NES (advanced via the femoral access) in the RA, providing countertraction to the traction applied from the superior approach. The enhancement and balancing of forces on the targeted lead by the combined ‘superior–inferior’ approach provides the strongest rail for the dissection tool.39 This allows the sheath to safely navigate angulations of the vasculature, especially at the innominate-SVC junction.38,40 The use of the NES to grasp the lead also transfers the traction force applied superiorly from the heart to the snare, reducing the risk of myocardial invagination injury as the dissecting sheath advances.
The tandem technique is effective and safe. In a retrospective series of 131 patients (267 leads), complete lead success was achieved in 96.2% of patients, with a major complication rate of 3.8% and 0% mortality; there was no SVC injury.37 The technique is particularly advantageous for leads of a prolonged dwell time. In a series of 40 cases (75 leads) with an average lead dwell time of 150 months, clinical and complete success was achieved in 98 and 92% of cases and leads, respectively.38 Lead age is a determining variate of procedural success.16,29 Encapsulating tissue around the leads over time can become dense and calcified, resisting dissection and, therefore, increasing the probability of procedural failure.29,41 The tandem procedure could help powered sheaths to overcome these challenges by providing a sturdy rail for the tool to track, thereby focusing the dissecting energy over the lead as it advances. The balancing of the superior and inferior forces also pulls the lead away from the lateral wall of the SVC, improving the geometric relationship between the dissecting sheath and the vessel, thereby reducing the risk of SVC injury.37,42
The application of the technique is limited in its anatomic scope; once the sheath reaches the right atrium, the lead is released from the NES to permit advancement of the sheath towards the lead tip. Injury to the tricuspid valve or the RV can occur from this point onwards. Muhlestein et al. reported three pericardial effusions occurring in their large series, most likely from RV injury.37 A combined approach of the tandem with the jugular pull-through has been used effectively in a small number of cases to overcome the final angulation existing between the RA and RV. Transfer to the jugular effectively straightens this angle, particularly for leads implanted at the RV apex.34,36
Other Tools
The forces applied to the lead permit it to act as a rail for the safe advancement of dissecting sheaths. Locking stylets provide control and stability of the targeted lead, permitting the operator to regulate the degree of traction being applied and, therefore, direct the countertraction by the extraction sheaths.
The two main stylets in current use are the Liberator (Cook Medical) and the Lead Locking Device (Philips), which ‘lock’ within the lumen, allowing a grip over the lead. The locking mechanism of the Liberator is targeted to the distal tip of the lead while the Lead Locking Device is designed to lock at multiple sites along the lead.43 With the stylet locked in place, more traction can be applied to the lead, providing a firm rail.39 Therefore, it is imperative that the stylet occupies the full length of the lead lumen, allowing traction to be transferred as far as the lead tip. This increases the resistance of the lead to the extraction forces, ensuring that the binding tissue gives way before the lead does.44 If the traction forces were to be applied directly to the lead, without a locking stylet, the lead components would fail at a lower load.39
An ex vivo simulation study demonstrated the effects of load bearing on pacemaker leads with and without locking stylets. Without a locking stylet, the tensile strength of the lead is based on the insulation material and its elasticity; with increasing extraction forces, the lead deforms and fails. With a locking stylet, the initial traction load is carried by the locking stylet, and when this fails, the lead bears the load. The combination of a locking stylet and lead increases the resistance to the extraction forces in comparison with the lead alone.44
The structure of the lumenless lead varies significantly compared with the stylet-driven leads; there is a core conductor in place of the lumen, which is wrapped by insulation and an outer conductor. As the lumen in a standard 7 Fr pacing lead composes almost half the diameter, the lumenless lead is considerably smaller.45 During TLE, the core cable of the lumenless lead can mimic the role of a locking stylet with the advantage that it spans the entire lead body, remaining attached to the distal screw and proximal connector. An ex vivo bench test study found that this engagement fortified the rail for a dissecting sheath, although the rail strength remained comparatively lower than a locking stylet. Mechanically, similar to the locking stylet, the inner core of the lumenless lead bears the extraction forces exerted on the lead in the beginning. Upon failure of this component, the lead stretches, relying on the tensile strength of the insulation and remaining conductor to provide a rail for the dissecting sheath.45
To further support lead integrity and thereby the rail, the lead can be reinforced by compressing all its components closely together. Currently, there are two methods for this: the use of a ligation suture with durable material or the application of the OneTie compression coil (Cook Medical), which wraps around the lead, ‘compressing’ all the lead apparatuses together. Ex vivo simulation studies have demonstrated that lead failure occurs in stages when exposed to extraction forces; each component has its own failure limit.39 By binding these components closely together, the tensile strength of the lead is theoretically improved. The degree of benefit lead compression provides is yet to be fully determined.
A bench-test study evaluating the effects of traction on lead integrity with a locking stylet and compression coil found that the compression coil provided minimal additional resistance to lead failure; the benefit was provided by the locking stylet. However, the authors did not evaluate the tensile strength of the lead with only the compression coil deployed. Importantly, the authors highlighted that there was retraction of the locking stylet with traction, which was significantly stabilised by the application of a compression coil.44 Consequently, the authors concluded that the binding of the lead components together using a compression lead provided better control of the lead with a fully deployed locking stylet, maximising the benefits of the stylet.
The bulldog lead extender (Cook Medical) is a simple, yet effective, tool; it is a metal wire with an end loop through which a lead can be threaded. Designed to grasp leads that are unable to accommodate a locking stylet, it is used to apply traction on the lead during extraction. In the event of lead disruption, the bulldog can be deployed to ‘extend’ the lead, provided it is externalised enough to be grasped. An extraction sheath can subsequently be advanced over this to dissect the lead free.46
The laser and rotational tools are complemented by outer sheaths: the VisiSheath (Philips) and the SteadySheath (Cook Medical). These provide crucial support to the powered sheaths, enhancing their dissecting capabilities and stabilising the tool when meeting resistance from highly calcified adhesions. Used in a piston action, the larger outer sheath can also dissect; it is able to engulf a lead with a broadening diameter from material snowploughing by the powered tool. The outer sheath can also be used to preserve access for new implants and for advanced extraction techniques.
Complications
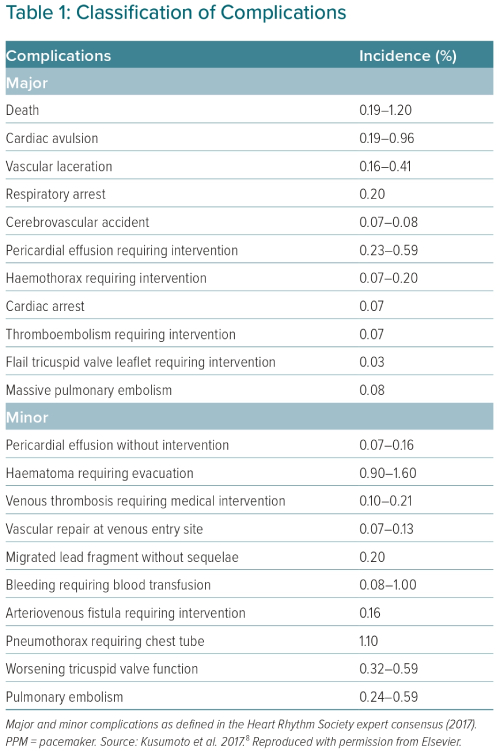
Invasive procedures carry a complication risk irrespective of the degree of complexity involved.47 The complications associated with TLE are classified within a minor or major category (Table 1). Any complication considered life-threatening, causing a disability or requiring a significant intervention is classified as major, while a minor complication is an undesired event that may require a minor intervention and does not lead to disability or death.6
Despite the complexity of the procedure, large series have reported a major complication rate of 1–1.7% in TLE, while procedural mortality has been found to be between 0.18 and 0.5%.5,16 This is comparable with other cardiological procedures, including percutaneous coronary intervention.48 Injury to the SVC is probably the most significant complication, which can quickly lead to death without surgical intervention. Even with intervention, mortality remains close to 50%.49 SVC injury most commonly occurs in the main SVC body – the portion that a dissecting sheath is directed towards as it enters the SVC from the innominate vein.50 This site is commonly extrapericardial and will lead to a right haemothorax with exsanguination of blood at a rate of ~500 ml/min;51 detection of the injury may not occur until haemodynamic shock develops. Conversely, myocardial injury is intrapericardial and leads to a pericardial tamponade. There is an almost immediate haemodynamic compromise, resulting in a much earlier revelation of the injury.50
An underestimated complication of TLE is the injury to the tricuspid valve (TV). This risk of TV injury begins with the lead implant in the RV. There is risk of valve perforation, impingement, laceration, entanglement or damage to the chordal structures. With time, the lead can also become tethered to the valve apparatus (Figure 5), compounding the risk of TV injury during the TLE. It has been suggested that RV leads implanted in the apical position are most likely to become tethered to the posterior valve leaflet, while leads situated between the septal-posterior leaflets are associated with significant tricuspid regurgitation (TR).52 The presence of significant TR is associated with adverse outcomes. A systematic review of 70 studies found that severe TR was an independent variate associated with an increased all-cause mortality risk.53 Data on the prevalence of TV injury post-TLE are limited, probably because the awareness of this complication has risen recently. A large observational study of 2,631 patients analysed the effect of TLE on TR. The authors found that TLE resulted in worsening TR in 9.69% of cases, while only 2.54% were classified as severe; 90.31% of cases did not experience any deterioration of TR, indicating no significant injury.54 Unsurprisingly, lead dwell time and the presence of firm connective tissue binding the lead to the valve apparatus were associated with development of severe TR; both variates increase the complexity of the procedure and commonly require large, powered sheaths to free the leads from the TV, which invariably increase the risk of injury.54
Acute Management of Complications
In the event of a major cardiovascular injury, stabilising the patient in preparation for emergency surgical repair is critical, and there are two methods for this. An endovascular occlusion balloon has been demonstrated to be an effective bridging strategy. The balloon is inflated in the SVC to occlude venous flow, buying precious time for the cardiac surgeons to locate and repair the injury.
An observational study of 116 patients with SVC injury during TLE evaluated the outcomes of occlusion balloon deployment against not using one. The authors found that in the balloon group, 88.2% survived, while among patients in whom the balloon was not used, 56.9% remained alive; occlusion balloon deployment reduced the odds of in-hospital mortality (odds ratio 0.13; 95% CI [0.04–0.40]; p<0.001).55 The benefit of the occlusion balloon is, however, limited to an extrapericardial injury of the SVC. The uncontrollable bleeding into the third space (hemithorax) could be halted through occlusion of the vessel using the balloon; without the occlusion, haemorrhagic shock is inevitable. The balloon is rendered ineffective for myocardial injuries, as the bleeding is contained within the pericardial space leading to a pericardial tamponade; this complication requires an immediate pericardiocentesis to bridge towards emergency surgery.
Percutaneous cardiopulmonary bypass is an alternative approach to achieving stability in patients with SVC laceration.50,56 A femoral-femoral bypass circuit can be achieved quickly, within minutes of a SVC injury – provided that femoral access has already been secured from the procedure onset; modern TLE procedures use the femoral vein routinely in a multi-access approach.34,37,38 There are other advantages of this approach. The bypass system can be used to maintain volume to reduce ischaemic injury to the vital organs, including the brain and kidneys. Having CPB established before initiating emergency surgery may be advantageous to the surgeon for performing repair in a controlled manner; off-pump surgical repairs are rarely performed in an emergency.50 Percutaneous CPB is particularly useful in patients with prior sternotomies in whom surgical access can be time-consuming owing to adhesions; the majority of SVC injuries can be repaired via a right-sided anterior thoracotomy in the second intercostal space, especially if there is a right haemothorax.50
Longer-term Outcome
Patient survival is the central objective of all procedures. Although procedural mortality is the metric by which extraction outcome is usually judged, 30-day mortality is a more important marker, as many deaths occur in this period. This endpoint includes procedure-related death, but more often highlights mortality related to the complex patient cohort. Thirty-day mortality is low in TLE, ranging between 1% and 3.4%, in the context of infection indication, which accounts for ~50% of patients.5,13,16,57,58
Infection is the most consistent variate identified for carrying 30-day mortality risk. Electra, the largest TLE study to date (n=3,555), reported that sepsis was associated with an almost fivefold increase in the odds of all-cause mortality (OR 4.93; 95% CI [2.72–8.93]; p<0.0001).16 This was echoed by PROMET, the second largest TLE study to date (n=2,205), while Brunner et al. identified infection as a predictor of 30-day mortality in their predictive model.58,59 This risk is significant enough to extend to >1 year after the TLE, but can be reduced with the appropriate management.13,60 Deharo et al., in their study of 197 CIED infection cases matched with 197 non-infection cases, found that TLE combined with the appropriate postprocedural care reduced the longer-term mortality risk associated with infection to match patients with non-infectious indications.60
Age is another variate affecting 30-day mortality. A nomogram created from a large study of 2,999 consecutive TLE procedures found age as a significant variate associated with 30-day mortality. This was validated by a PROMET substudy of 2,205 patients (3,849 leads) using propensity score matching, which found that age (≥80 years) was associated with 30-day mortality, but did not predict procedural outcome.29 It has been accepted that TLE as a procedure is safe across all age groups, despite the higher number of significant comorbidities in the older patient cohort.61 However, the postprocedural period may be more significant for the older cohort, as recovery can be affected by frailty; frailty is more prevalent with advancing age and is a recognised risk factor of postprocedural adverse outcomes.62
Conclusion
Transvenous lead extraction has evolved significantly over a short period of time. It is a life-saving procedure with an excellent safety profile. This is partly related to the development of powered sheaths, which have permitted the disruption of complex scar tissue around the leads, as well as the innovation in techniques. Despite this, the concept of traction and countertraction has remained central to all TLE methods; however, the expanded usage of the femoral and jugular approaches has provided an extra dimension with geometric advantages. Although there has been significant progress in TLE, further work is required, particularly in the pre- and postprocedural management; this is increasingly relevant with an ageing population living with complex medical needs.