Aortic stenosis (AS) is one of the most common valvular diseases in Western countries. AS is a degenerative disease and is therefore linked to age. The prevalence of severe AS is >7% among those aged >80 years,1 and the number of patients with AS will probably increase in coming decades due to the aging of the population.2 Progressive valve obstruction occurs during a long latent phase, with the patient remaining asymptomatic until the severity of the obstruction results in an inadequate heart function leading to symptom onset. Once symptomatic, severe AS has a poor prognosis, with a 12-month survival rate of approximately 65% among unoperated symptomatic patients.3,4 To date, there are no medical therapies that have been proven to delay the progression of AS or to correct valve degeneration. Aortic valve replacement (AVR; either surgical or percutaneous) remains the only treatment that has been demonstrated to improve survival.5–7
Current clinical guidelines recommend AVR when symptoms appear or left ventricular (LV) dysfunction occurs.8,9 However, management of asymptomatic severe AS remains a matter of controversy, and earlier AVR in certain scenarios is being increasingly supported by some groups. The aim of this review is to summarise the current evidence regarding the management of patients with asymptomatic AS.
Current Guideline Recommendations
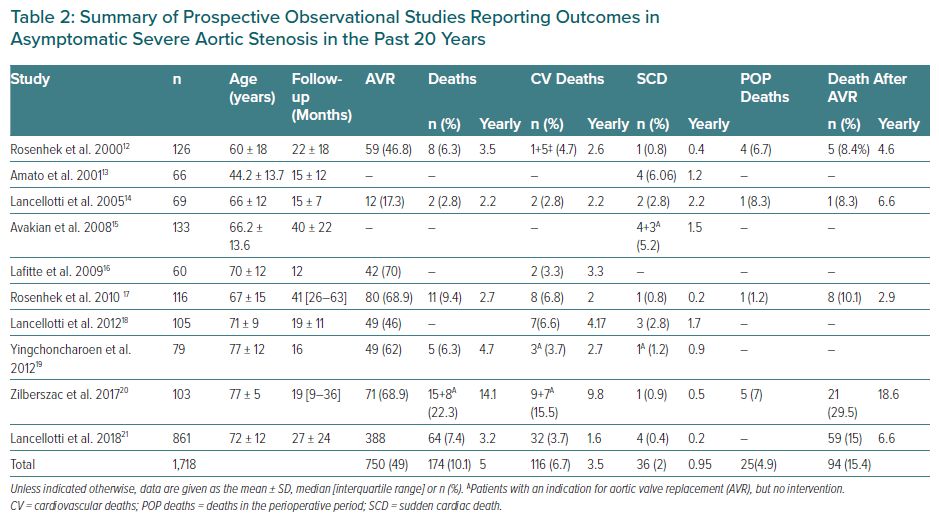
European and US guidelines present similar recommendations about AVR is indicated in asymptomatic patients that are based on three scenarios:8,9
- abnormal LV ejection fraction (LVEF);
- abnormal exercise test (with symptom development or a fall in blood pressure below baseline); and
- low surgical risk and the presence of high-risk criteria, namely very severe AS (defined as aortic peak velocity >5.5 m/s by the European guidelines and >5.0 m/s by the more recent US guidelines), a rate of peak transvalvular velocity progression >0.3 m/s per year or repeated and markedly elevated B-type natriuretic peptide (BNP).8,9
The European Society of Cardiology (ESC) guidelines also acknowledge severe pulmonary hypertension (systolic pulmonary artery pressure >60 mmHg confirmed by invasive measurement) with no evident explanation, whereas the US guidelines grant a 2b recommendation to progressive decreases in LVEF to <60% on three or more serial imaging studies.8,9 The ESC guidelines emphasise that all the recommendations regarding early intervention in asymptomatic AS relate to surgical aortic valve replacement (SAVR), but the US guidelines consider both SAVR and transcatheter aortic valve replacement (TAVR) in the case of systolic dysfunction, but only SAVR in the other indications (Table 1).8,9
It is important to note that there are low levels of evidence for all these recommendations. The recommendations are based on small, single-centre, retrospective, observational studies or expert opinions. Most of the situations identified as high-risk criteria are based on studies in which the primary end-point usually included the development of symptoms or undergoing AVR. It is crucial to understand that the evidence shows that all these factors are markers of the progression of the disease, but it is not clear whether early AVR in these scenarios will improve patients’ prognosis.
Natural History of Patients with Aortic Stenosis
Ross and Braunwald published a review of AS in 1968 that has traditionally been accepted as the natural evolution of AS: survival was excellent during a latent period when increasing obstruction and myocardial overload were occurring.10 Then, when symptoms appeared (angina, syncope and/or heart failure), survival rapidly decreased. This scheme represented the evolution of AS that was predominantly rheumatic in origin, with the age at the time of symptom onset being <60 years and the average age at the time of death being 63 years.10 Therefore, it does not necessarily represent the natural history of AS nowadays. Currently, most AS patients in the Western countries are older, usually have degenerative aortic disease and often present with significant comorbidities.11 Despite the extensive research on the field, the current natural evolution of AS is difficult to evaluate.
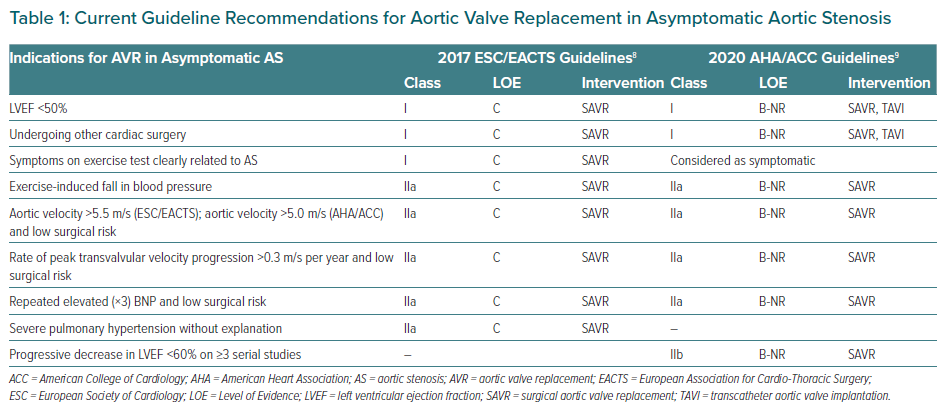
Many groups have published observational studies about the natural history of AS, but most have combined death and AVR as an event. The approaches to symptom evaluation and patient follow-up differ widely among the studies, as does the way to report death, so it is difficult to reach solid conclusions based on these studies. The main results of these studies are summarised in Table 2.12–21
Most of the events reported in these studies are the development of symptoms or AVR. Approximately half the patients required intervention, with total and cardiovascular mortality approximately 5% and 3.5% per year, respectively. The rate of sudden cardiac death is approximately 0.95% per year, as described previously.13,18,19 A recently published meta-analysis on the natural history of AS reported similar results: a rate of all-cause death of 4.8 per 100 patients per year and a rate of cardiovascular death of 3.0 per 100 patients per year, with sudden death occurring at a rate of 1.1 per 100 patients per year.22
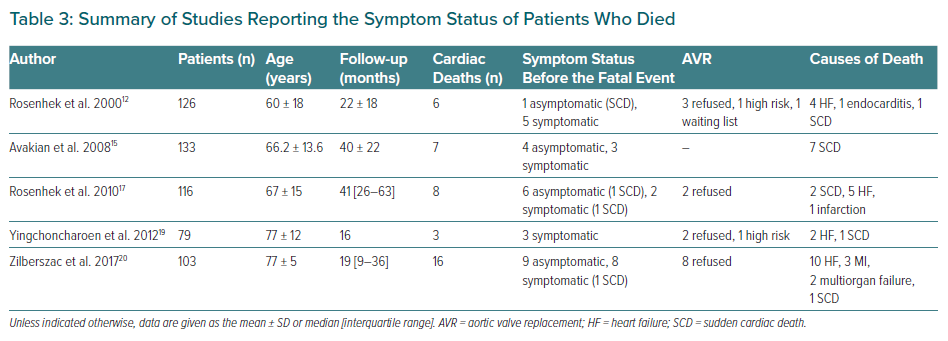
Some studies have reported on the symptom status of patients who died and the reasons for not performing AVR.12,15,17,19,20 There is considerable disparity here, with the rate of patients who remained asymptomatic until the fatal event ranging between 0% and 75% (Table 3). However, because some patients developed symptoms before death, these figures cannot be interpreted as mortality in asymptomatic patients with AS.
Only a few studies have reported deaths after AVR: the mortality rate within the perioperative period was reported to be 4.9%, increasing to 15.4% during the entire follow-up period.12,14,17,20,21 Of these studies, that of Rosenhek et al. in 2010 included only patients with very severe AS (peak velocity >5 m/s), although the results are similar to those reported by the other studies.17
In their study, Zilberszac et al. only included patients >70 years of age.20 As a result, mortality in that study is notably higher in both patients under clinical vigilance and after intervention.20 The effect of age on the natural history of AS and on the results of interventions performed in AS patients must be addressed because, as Table 2 clearly shows, the mean age of these patients is increasing.
The rate of progression of the disease is highly variable among subjects and difficult to predict.23 On average, aortic peak velocity is estimated to increase 0.2–0.3 m/s and aortic area to decrease 0.1 cm2.24,25 Symptom onset in patients with severe AS is likely to occur within 2–5 years.9 In the meta-analysis of Gahl et al., the yearly rate of patients who developed symptoms was 18.5%.22 As soon as symptoms appear, the prognosis of severe AS is very poor, with survival rates of only 15–50% at 5 years.8
Until now, symptom onset had been considered as the essential moment in the evolution of AS. We know that prognosis significantly worsens at this point, and we wait for it to occur before intervening in patients. However, as the complication rates of both SAVR and TAVR have decreased with time, there has been increasing concern about the development of irreversible cardiac damage that would not be corrected or reduced by later AVR and that may precede symptoms. Symptoms are subjective in nature and, so, basing treatment decisions on what the patient reports can be deleterious. Assessing the symptomatic status of a patient with AS is complex: on the one hand the disease progresses slowly and patients may adjust their activity gradually and unconsciously. On the other hand, symptoms may be non-specific: these patients are typically old, deconditioned and often present with comorbidities, such as pulmonary disease or obesity. Attributing the dyspnoea to AS can be challenging in many cases.
In addition to the symptom status of a patient, given the increasing age of the AS patient population, comorbidities play a very important role in the clinical evaluation of and decision-making for these patients. Guidelines recommend against performing AVR in patients with severe comorbidities if it is unlikely that the patient’s quality of life or survival will improve. A multicentre prospective registry of patients with AS has documented the effects of comorbidities on the presentation and management of patients.26 In that study, 50% of patients with severe AS had any kind of comorbidity, and the presence of comorbidities was associated with a greater likelihood of being symptomatic. The authors also reported that certain comorbidities, such as chronic kidney disease, appear to act as deterrents for indications for surgery, whereas the presence of ventricular dysfunction seems to be an incentive. TAVR is more frequently indicated in comorbid patients, and an active decision not to treat was more frequently chosen in the subgroup of patients with a higher comorbidity burden.26
Sudden cardiac death (SCD) can occur in asymptomatic patients with severe AS; in fact, SCD can be the first clinical manifestation of the disease.12–15,17,19,20,27,28 The estimated annual risk of SCD in clinically asymptomatic patients with severe AS is around 1%. Similar results are presented in Table 2 for data from prospective observational studies. Whether this low rate of sudden death would be reduced with early AVR is unknown. Although traditionally SCD has been linked to the severity of the stenosis, the exact mechanism that ultimately leads to SCD is unknown; patients with AS often have associated coronary artery disease. Moreover, hypertrophy and myocardial fibrosis are common in these patients and are known causes of tachyarrhythmias. In fact, it has been reported that SCD also occurs in patients with mild and moderate AS with an annual incidence of 0.39%/year.29 In these patients, sudden death has been related to LV hypertrophy, but not to stenosis severity.29 Moreover, the risk of SCD does not completely disappear after AVR, and SCD has been described as the second cause of cardiac death after both SAVR and TAVR.30 In a large contemporary register of 3,726 TAVR patients investigating cardiac death after the intervention, SCD occurred in 57 patients after a mean follow-up of 22 ± 18 months (5.6% of deaths, 16.9% of cardiac deaths).31 In that study, LVEF ≤40% and new-onset persistent left bundle branch block were identified as independent predictors of SCD, especially if the QRS duration was >160 ms.31 Unfortunately, no significant differences were observed in survival rate among patients who did and did not receive a prophylactic pacemaker, although this could be related to the small number of patients in this subgroup with new-onset persistent left bundle branch block who received a pacemaker.31
High-risk Markers in Asymptomatic Aortic Stenosis
The uncertainty about the best management in asymptomatic AS has led researchers towards the identification of patients at high risk of developing complications. These factors can be considered to be predictors of event-free survival, but, again, it must be pointed out that in most cases the predominant event analysed in the studies was the development of symptoms requiring intervention. Therefore, currently there is no evidence that AVR in these scenarios will improve outcomes in asymptomatic patients.
Echocardiographic High-risk Markers
Very severe AS, defined as aortic peak velocity >5 or >5.5 m/s depending on the study, has been defined as a high-risk marker in many studies. At aortic peak velocity above 5 m/s, the rate of symptoms onset is 50% at 2 years.9 Peak velocity is one of the strongest independent echocardiographic predictors of adverse cardiovascular events in patients with AS.12,19,25,27,32–35 In a recent prospective cohort of 1,375 patients with asymptomatic AS, among patients with a peak velocity >5 m/s, the risk of cardiovascular mortality was sixfold higher and the risk of postprocedural mortality was higher for those patients who underwent AVR.21 The very first published randomised control trial (RCT) comparing early surgery or conservative care in asymptomatic AS was performed in patients with very severe AS (defined as an aortic valve area of ≤0.75 cm2 with either peak velocity >4.5 m/s or mean gradient >50 mmHg).36 Although that study has several limitations that are addressed below, the authors reported a better prognosis in the early surgery group (death for any cause 7% in early surgery versus 15% in conservative care; HR 0.33; 95% CI [0.12–0.90]).
In some studies, a reduction in LVEF was an independent predictor of events.15,37–39 Current clinical guidelines recommend surgery when LVEF is <50%.8,9 However, patients with AS often have concentric hypertrophy and, in these cases, LVEF may substantially underestimate the degree of LV systolic dysfunction. In a large register of more than 900 subjects, LVEF <50%, as well as LVEF between 50% and 59%, was independently associated with poorer outcomes compared with LVEF ≥70%.40 Another retrospective study found that mortality was higher for patients with LVEF 50–59% than >60% after AVR.41 In addition, up to one-third of patients with AS and LVEF >50% had subclinical ventricular dysfunction, identified by speckle tracking echocardiography.42 All these data suggest that a cut-off of LVEF <50% lacks sensitivity to identify subclinical ventricular systolic dysfunction and that patients with an LVEF between 50% and 59% should be monitored closely.
The rate of progression in peak velocity is individual and difficult to predict. The normal rate is estimated to be around 0.3 m/s per year. Some studies have reported that a higher rate of progression is associated with a higher risk of events.12,24,32 Rapid progression (≥0.3 m/s per year) predicted excess mortality (versus a slow progression rate) after adjustment for other important risk factors, such as LVEF or peak aortic velocity, in a recent retrospective study.43
Global longitudinal strain (GLS) is an early marker of abnormal contractility and is more sensitive than LVEF in identifying subtle abnormalities in myocardial function; in addition, GLS abnormalities are related to myocardial fibrosis.44 Although the optimal scenario for GLS analysis and cut-off values are still under investigation, there is growing evidence of the added value of GLS in patients with severe AS and a normal LVEF.27,45,46
Exercise Stress Test
The incidence of an abnormal exercise stress test in patients with asymptomatic AS is approximately 50%.23 Developing clearly valve-related symptoms during the stress test is considered a Class I indication for AVR in both European and US guidelines, and an abnormal pressure response is a Class IIa indication for AVR. Studies investigating exercise tests in asymptomatic AS are very heterogeneous regarding the exercise protocols and the criteria to be considered abnormal. Most studies have shown that an abnormal test is an excellent predictor of developing spontaneous symptoms in the future, but there has been no clear relationship shown with mortality.14,16,33 A classic study reported that the stress test may identify patients at risk of SCD,13 but these results have not been confirmed in other studies.
Exercise stress testing may help uncover symptoms, revealing them in up to 38% of patients with asymptomatic AS.47 However, it has to be noted that the positive predictive accuracy for exercise-induced symptoms is limited in patients aged >70 years.33
Biomarkers
Serum BNP concentrations are predictive of symptom onset during follow-up, but also of persistent symptoms after AVR.48 A recent observational study demonstrated that elevated BNP levels were associated with an increased risk of death or hospitalisation for heart failure.49 Based on this evidence, the new US guidelines have changed and now recommend AVR (Class IIa indication) with a BNP level >300 pg/ml (threefold normal). Remarkably, asymptomatic patients with BNP concentrations <100 pg/ml had an event rate of only 2.1% at 1 year.9,49 Although the true value of BNP has to be tested in an RCT, it seems reasonable to integrate BNP concentrations in the clinical assessment of patients with asymptomatic AS, particularly for the identification of lower-risk patients who can be followed periodically.
The BNP ratio is calculated as measured BNP/maximal normal BNP value for age and sex, and represents BNP activation.50 In a large cohort of 1,953 consecutive patients with at least moderate AS, the BNP ratio was an independent predictor of mortality.50 In the subgroup of patients with severe asymptomatic AS and a normal LVEF, a BNP ratio >1 independently predicted survival.50
Cardiovascular MRI
In AS, progressive narrowing of the valve causes chronic pressure overload of the LV. This triggers a hypertrophic response able to maintain myocardial performance for a long time. This ventricular remodelling is also accompanied by the development of myocardial fibrosis, myocyte injury and adverse remodelling of the extracellular matrix.51 Histological studies have shown that higher degrees of myocardial fibrosis at the time of AVR are associated with worse long-term survival after the intervention.52 Advances in cardiac imaging allow a precise non-invasive quantification of myocardial fibrosis with cardiac magnetic resonance (CMR). Two different patterns of myocardial fibrosis can be identified by CMR, namely diffuse interstitial fibrosis and replacement fibrosis.
Diffuse interstitial fibrosis is a reactive and at least partially reversible process. It is assessed by novel CMR T1 mapping approaches and it has been demonstrated to improve after AVR.53,54 High native T1 values on non-contrast T1 mapping have been shown to be an independent predictor of mortality and hospitalisation for heart failure in patients with significant AS.55 Replacement fibrosis is a later phenomenon and is irreversible. Replacement fibrosis is assessed by late gadolinium enhancement techniques and is relatively common in patients with AS (found in 20–66% of patients undergoing CMR).51 Late gadolinium enhancement does not regress after AVR, decreases the chances of improvement of LVEF and has been found to be an independent predictor of all-cause mortality after the intervention.52,56
It seems clear that myocardial fibrosis detected by CMR is frequent in patients with severe AS and is an adverse prognostic indicator. These findings raise the question of whether long-term outcomes would be improved if AVR is performed before adverse LV remodelling has occurred.
Cardiac CT
Multislice cardiac CT allows the severity of aortic valve calcification to be quantified with high accuracy. In asymptomatic AS, the calcium score of the aortic valve correlates strongly with clinical outcomes, such as symptom development, death or AVR.57,58 Recent research has demonstrated that the extracellular volume, a parameter of diffuse myocardial fibrosis, can be assessed by cardiac CT, with a good correlation with histology and CMR measurements in patients with AS.59 Furthermore, in patients with severe AS undergoing CT before AVR, quantification of extracellular volume fraction was correlated with functional status and predicted a composite of adverse clinical outcomes after the intervention.60,61 Although its value in patients with asymptomatic AS has to be proven, cardiac CT is worthy of further investigation given the need for cardiac CT imaging prior to TAVR and the utility of calcium scoring as a marker of stenosis severity in cases of doubt.
Early Aortic Valve Intervention Versus Watchful Waiting
Determining the optimal timing of AVR in patients with AS depends not only on the severity of the valvular lesion, but also on the safety, efficacy and long-term results of the procedure to be applied. An early intervention while the patient is asymptomatic exposes the patient to both procedural complications (which can be fatal, but also create disabilities) and long-term complications (bleeding, embolism, paravalvular leak, endocarditis). In addition, the earlier the intervention, the higher the probability of a reintervention in the future due to prosthesis degeneration. Conversely, postponing AVR confers a low but real risk of suffering a life-threatening event and a risk of developing irreversible structural damage in the heart that would worsen the prognosis after the intervention. In addition, the risk of the intervention itself will be higher if the patient gets older.
In the quest to determine whether early AVR benefits patients with asymptomatic severe AS compared with the current recommendations (clinical vigilance and AVR if the patient becomes symptomatic or the LVEF decreases below 50%), an RCT and several meta-analyses have been published recently.
The RECOVERY trial randomised 145 patients with asymptomatic very severe AS to early surgery or to conservative care.36 The cardiovascular mortality rate after a median follow-up period of 6 years was 1% in the early surgery group and 15% in the conservative care group. Several aspects regarding that study deserve to be mentioned. Patients >80 years of age were excluded, the mean (±SD) age was 64±9 years and more than half the patients had a bicuspid aortic valve, so this population differs considerably from what a real clinical scenario of severe AS represents nowadays.36 Probably due to this selected population, operative mortality was zero and the mortality in the follow-up period was also strikingly low (7% of all-cause mortality). These figures are far from the 5% and 15%, respectively, reported in the observational studies (Table 2). The small number of deaths represents a statistical limitation of the RECOVERY trial. The surgical outcomes reflect the surgical excellence of the participant centres, but the results may not be extrapolated to less-experienced centres. It is also surprising that 22% of patients in the conservative arm never underwent surgery despite the long follow-up period.36 This reflects that patients with asymptomatic AS are a heterogeneous population in whom a one-size-fits-all strategy may not be the best approach.
Six meta-analyses have been published in recent years.2,22,62–65 All have included a variable combination of the same 12 studies: one RCT and some prospective and most retrospective observational studies. The meta-analysis published by Lim et al. showed a trend towards a reduction in mortality with early AVR, but no significant difference in cardiac mortality.62 The remaining studies found a significant benefit for AVR compared with conservative management, with lower all-cause and cardiovascular mortality rates.2,22,63–65 The results of all six meta-analyses are summarised in Table 4. Although all these studies point the same direction, their results must be analysed with caution. All showed a significant heterogeneity between studies; the meta-analyses are, of course, exposed to publication bias, but their main limitation is that their quality depends on the quality of the studies they included, many of which were retrospective studies. In addition, the stress test was not universally performed in the studies included, so there is no way to determine whether all patients were truly asymptomatic, and the follow-up of patients in the conservative group was not protocolised. In fact, in one of the studies included in all six meta-analysis, up to 30% of the patients developing symptoms in the conservative group during the follow-up did not undergo AVR.28 Thus, although the conservative strategy is often known as ‘watchful waiting’, we have no evidence that, in these cases, the waiting was watchful.
There are currently five ongoing RCTs (AVATAR [NCT02436655]; EARLY TAVR [NCT03042104]; EVoLVeD [NCT03094143]; DANAVR [NCT03972644]; EASY-AS [NCT04204915]) comparing the ‘wait for symptoms strategy’ with surgical or percutaneous AVR.66 Although all these RCTs will help us to better understand the role of AVR in asymptomatic AS, the most promising seems to be the DANAVR trial (NCT03972644). This trial is designed to evaluate patients with high-risk features: left atrium dilatation, diastolic dysfunction, abnormal GLS or elevated N-terminal pro BNP (NT-proBNP); the primary end-point is death and the estimated follow-up period is 60 months. We believe that, to adequately address early aortic valve intervention versus watchful waiting, a controlled trial should not include AVR or symptoms in the medical group as an outcome and that follow-up must be long enough to include both perioperative deaths and long-term deaths in the conservative arm. Disability and quality of life parameters should also be considered because they are a significant event in aged patients. Due to the specific features of patients with asymptomatic severe AS, individual life expectancy in good health should be the focus of the comparison between the different strategies rather than overall survival.
Role of Transcatheter Aortic Valve Replacement in Asymptomatic Patients
Current European guidelines do not recommend TAVR in asymptomatic patients, whereas US guidelines only recommend TAVR in the case of LV dysfunction.8,9 Although it stands to reason that TAVR is an interchangeable treatment choice to SAVR in asymptomatic patients, there is scarce scientific evidence in this scenario. Until comparable long-term durability is demonstrated, SAVR remains the first choice for lower-risk younger patients, at least from an academic point of view. However, real-world data show that with the emergence of percutaneous techniques in the treatment of AS, an increasing number of patients are undergoing TAVR while asymptomatic.67
A recent prospective multinational registry of patients with severe AS across Europe reported that, in the subgroup of 392 asymptomatic patients who were clinically evaluated to decide on treatment, AVR was decided on for 153 patients and, in 58% of cases, was performed percutaneously.67 In that registry, up to 66 patients underwent TAVR while asymptomatic and having no formal indication according to current clinical guidelines. As the technique improves and the complications of the procedure decrease, the indications for TAVR are expanding. TAVR will definitely play an important role in the shift towards earlier intervention in AS patients in the future, but, while we wait for scientific support for earlier indications, we should be cautious and try to avoid overusing percutaneous interventions, as has occurred with percutaneous coronary interventions over the past three decades.
Beyond the Aortic Valve: Role of Extravalvular Cardiac Damage Staging
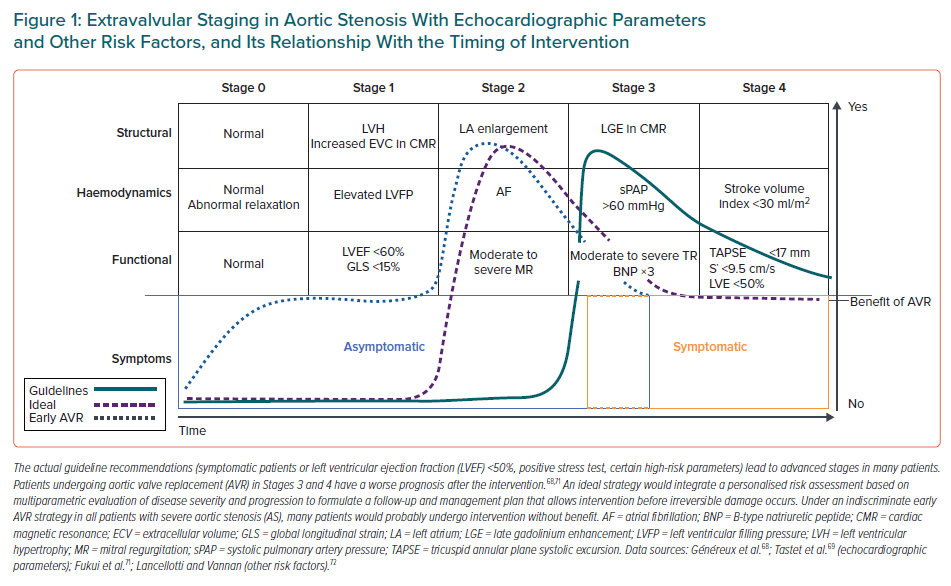
A novel staging classification for patients with AS has recently been proposed.68 This staging system is based on the extent of the extravalvular cardiac damage caused by the aortic stenosis as determined using echocardiography: Stage 0, no extravalvular cardiac damage; Stage 1, LV damage that includes significant hypertrophy or diastolic dysfunction and subclinical systolic dysfunction; Stage 2, left atrial or mitral valve damage; Stage 3, pulmonary vasculature or tricuspid valve damage; and Stage 4, right ventricular damage. This classification was predictive of 1 year mortality in patients undergoing AVR.68
The model was refined and validated in a cohort of patients with asymptomatic severe AS.69 In these patients, Stage ≥2 was an independent predictor of mortality. Notably, up to 60% of patients were in these advanced stages despite being asymptomatic. This classification has also been validated by other investigators.70,71
Because this staging system is based only on echocardiography, a variation to the system to include other markers of heart damage with prognostic association in AS, such as fibrosis in CMR or biomarkers, has been proposed.72 Figure 1 illustrates this way of staging and its relationship with the timing for surgery This constitutes a promising approach to the problem of asymptomatic AS: an individualised strategy focused on the repercussions of the disease in the heart, looking to intervene before there is irreversible damage to the structure and function, which may have an effect on patients’ prognosis not only before, but also after the intervention.
Conclusion
Patients with asymptomatic AS are a heterogeneous population in whom early AVR will surely have a role, but to date there is no evidence supporting changing the usual ‘wait for symptoms’ practice. However, this waiting must be active: close monitoring of patients is warranted and symptoms should be evaluated carefully, performing stress tests in case of equivocal or non-specific symptoms. It has been proven that symptoms are not a good marker of the status of the patient with severe AS; to improve patient assessment, many high-risk markers are being identified and will be tested in RCTs. While we wait for the results of RCTs to set the appropriate indications for early AVR, a shift in the paradigm from focusing on the valvular lesion based only on data from Doppler imaging to staging AS considering structural, haemodynamic and biomarkers abnormalities seems to be the best approach.