In women, cardiovascular disease (CVD) is the leading cause of death and, despite great strides in reducing mortality from CVD, the number of women dying because of CVD has increased recently.1 The increase in mortality has been attributed, in part, to the risk of death in younger women with CVD due to a lack of recognition and undertreatment of young women with CVD.2 CVD is also the leading cause of maternal mortality, accounting for one in three maternal deaths.3 The peripartum and postpartum risk of CVD mortality is only one part of the risk to a young woman’s health. Pregnancy also identifies women who may be at a greater risk of CVD, based on the development of adverse pregnancy outcomes (APOs), and, in essence, may identify women who could benefit from atherosclerotic CVD (ASCVD) risk reduction efforts. As such, APOs are considered as risk enhancers of ASCVD by the 2018 American College of Cardiology (ACC)/American Heart Association (AHA) guideline on the management of blood cholesterol and the 2019 ACC/AHA guideline on the primary prevention of CVD.4,5 Identifying women with such risk enhancers and addressing ASCVD risk has been identified as an important component of care in the ‘fouth trimester’, which should involve comprehensive care and risk assessment within the 12-week postpartum period.6
The risk of APOs is not rare, with approximately 30% of women experiencing an APO. This includes gestational hypertension (3–14% of births), pre-eclampsia (2–5% of births), gestational diabetes (5%), preterm delivery (6–12%) and the delivery of a small-for-gestational-age (SGA) infant (prevalence varies by country; Table 1).7 APOs occur more frequently in black and Asian women, who often present with more severe clinical presentations and have poorer outcomes.8–10 There are numerous observational studies showing a strong association between these APOs and CVD, including premature CVD.7,11–15 Identifying women with a history of APOs provides a unique opportunity to identify women at risk for CVD and initiate early primary prevention efforts in a higher-risk group before the onset of adverse cardiovascular events. The American College of Obstetrics and Gynecology and the AHA have proposed a new paradigm for postpartum care to identify women at risk for future CVD, aptly named the ‘fourth trimester’.6,16 The purpose of this paper is to discuss the effects of pregnancy and APOs on the risk of CVD.
Pregnancy as a Stress Test
Pregnancy is a physiological stress test to the heart. During pregnancy, there is an increase in circulating blood volume. This occurs in the setting of a reduction in systemic vascular resistance, lower blood pressure and increased cardiac output, which is essential for the optimal growth of the developing foetus.17 These adaptive changes are designed to provide adequate uteroplacental circulation, given the increased metabolic demands during the gravid state. Insufficient haemodynamic changes can result in significant maternal and fetal morbidity and mortality. In addition, even after these acute issues resolve, some studies have shown an association between APOs and hypertension, left ventricular changes, vascular dysfunction, chronic kidney disease and CVD after the reproductive years.12,18–23
Adverse Pregnancy Outcomes and Associated Adverse Cardiovascular Outcomes
Gestational hypertension and pre-eclampsia have been associated with a 2- to 4-fold increased risk of CHD, heart failure and stroke, with recurrent pre-eclampsia having the highest risk.24–26 Although the relative risk is highest within the first year postpartum, the risks persist decades after the pregnancy, when the absolute risks are greater than those immediately postpartum.25,26 Hypertensive disorders of pregnancy (HDP) are associated with accelerated cardiovascular aging, with a greater prevalence of subclinical atherosclerosis and arterial stiffness index among women aged >40 years.26,27 In addition, HDP have been associated with aortic stenosis and mitral regurgitation, as demonstrated by the UK Biobank cohort, showing that the CVD risk goes beyond the impact of just the development of chronic hypertension.26
Women who had gestational diabetes have up to seven- and twofold increased risks of developing type 2 diabetes and major cardiovascular events (independent of type 2 diabetes), respectively, than those without gestational diabetes.28,29 Studies have shown a 16–29% cumulative incidence of diabetes after 10–20 years of follow-up in women with gestational diabetes.30,31 Preterm delivery has been associated with a 1.4- to 2-fold risk of CVD, CHD and stroke.12,32 The highest risks occurred when the deliveries occurred before 32 weeks gestation or in medically indicated preterm deliveries.12
A recent study showed that at 5 years postpartum, the incidence of MI increased more rapidly in preterm than term deliveries, whereas for ischaemic stroke this occurred after 10 years.33 A recent meta-analysis did not pool studies on women with delivery of a SGA infant due to variations in the definition of SGA between the studies.34 However, the authors of that analysis noted a consistent trend of increased CVD risk in these women across all 10 studies included, with an effect estimate ranging between 1.09 and 3.50 and a follow-up period of up to 21 years.34
Mechanisms Driving the Association Between Adverse Pregnancy Outcomes and Cardiovascular Disease
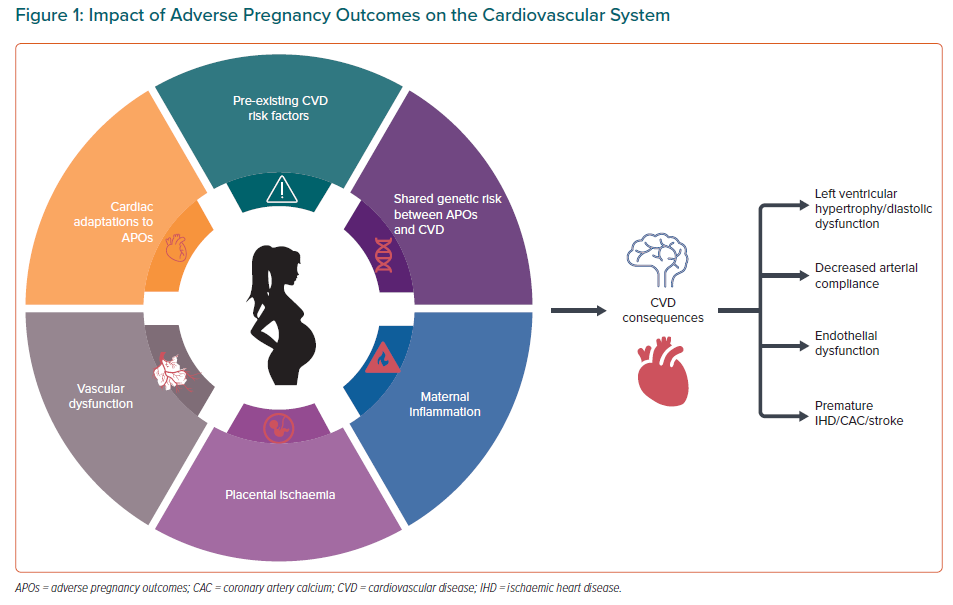
APOs are common and, although they are separate diagnoses, all these disorders seem to share an underlying pathogenesis, including placental ischaemia, maternal inflammation and vascular dysfunction.35–39 What is not clear is whether the APO itself initiates a pathway that results in CVD or whether the APO uncovers a woman’s predisposition to CVD (Figure 1). Regardless, APOs have immediate risks to maternal and fetal health, in addition to longer-term CVD consequences.7,12,40,41 As such, the occurrence of APOs provides an insight into a woman’s future cardiovascular health.
Systemic Endothelial/Microvascular Dysfunction
APOs appear to share similar metabolic and/or vascular abnormalities, which are reflected within the placenta. In a pregnancy without any APOs, the maternal spiral arteries widen after a trophoblast invasion, resulting in low-resistance blood flow in the uteroplacental unit.37 In contrast, in a pregnancy affected by pre-eclampsia, the trophoblast invasion is shallow, with inadequate spiral artery remodelling that results in poor perfusion of the placenta, placental ischaemia and oxidative stress. There is also evidence of inflammatory markers within the maternal blood in those with APOs, which are not seen in those with an uncomplicated pregnancy.37,42–44 Theoretically, the inflammatory state and the anti-angiogenic state could be the shared mechanisms by which APOs increase underlying CVD risk.45 Placental lesions have also been associated with cardiovascular risk factors.46–48 Increased soluble fms-like tyrosine kinase-1 has been associated with atherosclerosis.49
Furthermore, women exposed to pre-eclampsia may also have arterial stiffness or endothelial damage, which, in turn, are related to their increased long-term CVD risk.50,51 Women with spontaneous preterm delivery have been observed to have a proinflammatory phenotype, with higher C-reactive protein levels during pregnancy.52,53 It may be that the inflammatory processes associated with preterm delivery increase the risk of endothelial dysfunction and subclinical vascular disease, and consequently increase CVD risk in the future.52,54
In addition, because placental dysfunction may be due to vascular and endothelial cell dysfunction, women who have subclinical CVD phenotypes may not be able to mount an appropriate haemodynamic response in pregnancy. For example, placental growth factor, a hormone that promotes angiogenesis, is significantly reduced in pregnancies with pre-eclampsia, with or without SGA infants.55,56 The shared placental and maternal vascular characteristics in those with APOs support an overlapping pathophysiology for future CVD, regardless of how different the APOs appear in their presentation.
Cardiac and Coronary Changes
Structural changes in the myocardium can occur in women with APOs. In pre-eclampsia specifically, afterload-dependent cardiac remodelling can be seen that is similar to the remodelling of the myocardium seen in hypertension. Specifically, an increase in left ventricular wall thickness, particularly concentric left ventricular hypertrophy, has been seen even in those with mild gestational hypertension.57,58 Diastolic dysfunction and impaired left ventricular relaxation have been documented in patients with pre-eclampsia.58 Left atrial remodelling may also develop, most noted in preterm pre-eclampsia.59 These changes can also persist many years after the incident pregnancy.22,23
Furthermore, women with previous pre-eclampsia had higher carotid intima–media thickness, lower coronary flow reserve and higher high-sensitivity C-reactive protein values than those without pre-eclampsia.60 In addition, both pre-eclampsia and high parity number have been linked with accelerated atherosclerosis.27,61 Women with previous preterm births also have higher atherogenic lipids and carotid arterial wall thickening in the decade after delivery than women who had term births.62 For women with ischaemia without obstructive coronary artery disease, a history of APO is associated with lower coronary flow reserve suggestive of coronary microvascular dysfunction.63
Role of Cardiovascular Disease Risk Factors
APOs and CVD share ASCVD risk factors, and women with APOs have been shown to have a higher CVD risk factor burden, with a greater prevalence of hypertension, hyperlipidaemia, diabetes, kidney disease, obesity and tobacco use, in addition to a greater risk of developing these risk factors several years after the pregnancy.7,64–66 Except for tobacco use, which is inversely associated with pre-eclampsia, the other biochemical risk factors have been shown to persist in women years after HDP.67 Therefore, pre-eclampsia and gestational hypertension may be independent risk factors for future CVD because the post-pregnancy body may not fully recover from the damage to the vascular, endothelial and metabolic systems during pregnancy. With further insults to the body over time, the damage sustained during pregnancy may manifest in later life as cardiovascular events.68 Women with pre-existing cardiovascular risk factors, such as an adverse lipid profile and glucose status, are also at increased risk of preterm delivery.69 Similarly, delivery of SGA infants is linked to the development of maternal hyperlipidaemia, hypertension and increased calculated 10-year CVD risk prior to the onset of CVD.70,71 Nonetheless, the excess risk based on risk factors does not fully account for the amount of CVD seen women with APOs.72 Therefore, other mechanisms are likely to be involved in the association between APOs and CVD.
Other Mechanisms
Families of women with an APO also have increased risks of APOs and CVD, suggesting that underlying genetic factors may also contribute to the association.73,74 Women at risk of APOs and CVD may also have genetic mutations that are involved in the disease process. For example, there are maternal sequence variants associated with both pre-eclampsia and hypertension.75 Similarly, genetic predisposition to hypertension has been associated with HDP.76 Furthermore, an association was found between single nucleotide polymorphism variations in genes for cholesterol metabolism and preterm delivery.77
Conversely, multifactorial mechanisms may explain the association of APO with CVD, and socioeconomic factors have also been considered. For example, CVD and high parity number are both more frequently observed in low socioeconomic classes.78 High parity number is also associated with a small increased future paternal CVD risk.79–81 Because the observed associations in both mothers and fathers attenuated following adjustment for lifestyle factors, there may be residual confounding by socioeconomic class and/or lifestyle.80
Evolving Role of Adverse Pregnancy Outcomes in the Prediction of Cardiovascular Disease Risk
CVD risk assessment and stratification in women remain complex and, historically, have underestimated risk, especially in young women.82 Not only do traditional CVD risk factors such as hypertension, hyperlipidaemia and tobacco use, among others, need to be considered, but additional sex-specific factors, such as APOs, should also be included. However, existing risk stratification schemes, such as the ASCVD Pooled Cohort Equations model, do not include pregnancy or other gynaecological history.82 Whether routine incorporation of pregnancy complications improves the ability to risk stratify women for CVD is unknown and has more recently been investigated. The HUNT study by Markovitz et al. assessed the long-term risk of CVD, including MI, CHD and stroke, in women without prior CVD history in approximately 18,000 subjects with a prevalence of APOs of 39%.11 In that study, of all APOs, only pre-eclampsia was associated with increased CVD risk. The inclusion of pregnancy complications only led to the reclassification of 0.4% of women without events into lower-risk categories and 2% of women with events were correctly reclassified into higher-risk categories.11 A similar study by Timpka et al. assessed HDP and low birth weight in subjects aged ≥50 years and found that the inclusion of these APOs did not significantly improve CVD risk prediction.83 In the US, Stuart et al. studied the role of HDP and parity in a cohort of women aged ≥40 years without CVD risk factors or history of CVD.24 HDP and parity were added to the ASCVD Pooled Cohort Equations model, and these variables were associated with elevated ASCVD risk independent of established CVD risk factors; however, the risk reclassification across risk groups or age stratification did not change: 0.6% of previously low-risk women who developed CVD were reclassified into a higher-risk group, but 8.3% of women previously classified as being of intermediate risk were incorrectly reclassified as low risk.24 Dam et al. compared CVD risk prediction in women with and without a history of HDP among the Framingham Risk Score, the Pooled Cohort Equations model and the Systematic Coronary Risk Evaluation (SCORE) model, and similarly found that none of the models was more predictive in women with than without a history of HDP.84 Although these studies do not demonstrate improved discrimination of risk with the inclusion of APOs, this may be explained by underlying embedded association of APO risk with traditional CVD risk factors such as hypertension and diabetes.
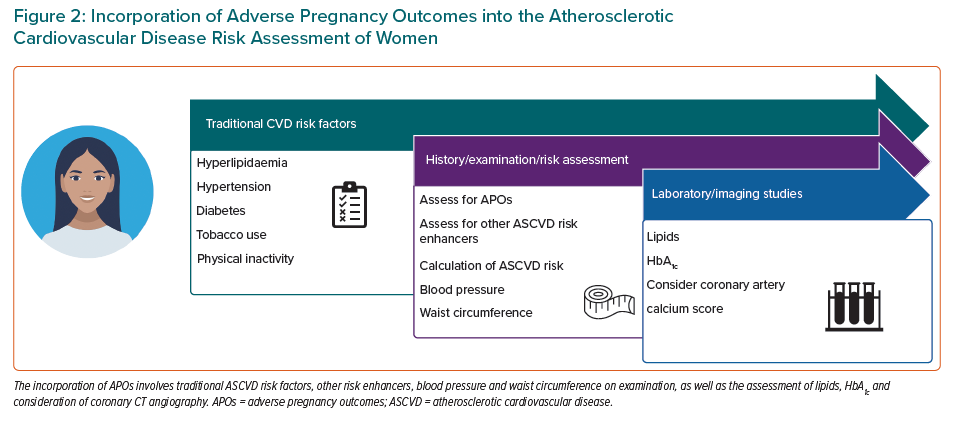
APOs serve as a ‘window’ into future CVD risk, either through development of traditional CVD risk factors and/or an independent association with underlying vascular dysfunction. As such, it is important to screen for APOs as risk markers to guide potential risk mitigation strategies. In 2018, the ACC/AHA cholesterol guidelines for the first time acknowledged APOs as CVD ‘risk enhancers’ for consideration of statin use in women with borderline to intermediate risk, as calculated by the Pooled Cohort Equation.4 The inclusion of APOs among other risk enhancers allows clinicians to personalise decision making regarding statin therapy beyond just generalised risk assessment. The ACC/AHA cholesterol guidelines also recommend the consideration of coronary artery calcium (CAC) assessment to help further guide decisions regarding statin therapy.4 The use of CAC to guide prevention therapies, such as statins, is particularly intriguing in women with a history of APOs.
Several studies have noted an association between coronary CT angiography (CTA)/calcium scoring in young women and a history of APOs (Figure 2). Benschop et al. found that, compared with women without a history of pre-eclampsia, pre-eclampsia is independently associated with CAC even when accounting for traditional CVD risk factors.85 Another study assessed a broader set of APOs in Black women, including preterm delivery, pre-eclampsia and gestational diabetes, against matched controls without APOs with regard to coronary CTA findings.86 In that study, any APO was associated with higher rates of atherosclerotic coronary disease, defined as ≥20% luminal narrowing disease and obstructive ≥50% luminal narrowing disease. That study was particular important because research in this area in Black women, who have high rates of pregnancy complications, is lacking. Whether coronary CTA should play a more routine role in assessing CVD risk in women with APOs is unknown and an area in need of further study.
Conclusion
Associations of APOs with future CVD have been reported in the literature. However, the underlying causal mechanisms remain unknown. It is important to raise awareness of the importance of these associations and the current recommendations among healthcare professionals, as well as among the women themselves. Further research is needed to elucidate the pathophysiology behind these associations. This, in turn, will inform future research in the role of ASCVD risk assessment and the effect of aggressive ASCVD risk modification on CVD outcomes in women with a history of APOs.