Hypertensive disorders of pregnancy (HDP) include a spectrum of complications, such as gestational hypertension (GH) and pre-eclampsia/toxaemia (PET), which affect 5–10% and 3–5% of pregnancies, respectively.1 The incidence of HDP has increased during the past two decades, and they still account for 10–22% of maternal mortality worldwide.2,3 Systemic cardiovascular impairment is a pathognomonic feature of the definition of HDP (de novo onset of systolic blood pressure [BP] ≥140 mmHg and/or diastolic BP ≥90 mmHg after 20 weeks’ gestation) and of the pathophysiology of PET, which is a multiorgan disorder secondary to endothelial dysfunction.1 Furthermore, these women are at substantially increased risk of cardiovascular disease (CVD) during pregnancy and following delivery – from the immediate postpartum period to several decades later. The association between HDP and CVD, as well as the opportunity to optimise postnatal maternal cardiovascular health, will be explored in the current review.
Cardiovascular Disease in the Peripartum Period
Women affected by HDP are at increased risk of acute cardiopulmonary complications in the peripartum period, with higher rates witnessed in women from ethnic minorities4,5 In a cohort of 11,304,996 pregnancies, Minhas et al. demonstrated that PET was associated with an increased risk of acute CVD in women of all races/ethnicities. Black women had the highest rate of PET and CVD, including peripartum cardiomyopathy, heart failure and arrhythmias. However, after adjusting for socioeconomic factors and comorbidities, the risk of cardiovascular complications was highest among Asian/Pacific women during hospitalisation for delivery.5
These acute cardiovascular disorders might manifest with clinical symptoms, such as dyspnoea, oedema and headache, that are common in pregnancies complicated by PET. Therefore, a thorough clinical evaluation and targeted investigations, including cardiac imaging and/or maternal biomarkers, might be indicated in women with HDP presenting with the aforementioned symptoms. The management of CVD in pregnancies with HDP should be guided by a cardio-obstetric multidisciplinary team, including an obstetrician, an anaesthetist and a cardiologist/obstetric physician.
Cardiopulmonary Disease
Cardiopulmonary morbidities include congestive heart failure, pulmonary oedema, acute respiratory distress syndrome, mechanical ventilation or cardiopulmonary arrest.6
The most common cardiopulmonary complication is pulmonary oedema, which accounts for up to 3% of PET cases in the antepartum or postpartum period. It is associated with significant morbidity and mortality. However, recent data suggest that mortality from pulmonary oedema has been reduced significantly by prompt diagnosis and aggressive management.7 Increased vascular permeability, decreased colloid osmotic pressure, left ventricular (LV) dysfunction, tocolytics and iatrogenic volume overload are the main components of pulmonary oedema in PET. The postpartum decrease in colloid osmotic pressure might explain why 70–80% of pulmonary oedema develops after delivery.8,9 The combination of LV diastolic dysfunction and increased vascular resistance increases hydrostatic forces in the pulmonary vasculature. Maternal age, obesity and pre-existing chronic hypertension (CHT) have been identified as the most common risk factors for pulmonary oedema.4 Although a rare complication, PET complicated by pulmonary oedema or other cardiopulmonary complications can advance to pregnancy-related acute respiratory distress syndrome associated with up to 44% of maternal mortality.10
Significant cardiac changes in LV geometry and function occur in women affected by HDP compared with normotensive pregnancy. Most diastolic indices are impaired in women with HDP compared with normotensive pregnancies.11,12 Vaught et al. showed that patients with severe PET had grade II diastolic dysfunction in 12.7% of cases.13 All abnormal diastolic findings can be associated with developing heart failure symptoms.14 Furthermore, significantly reduced LV global longitudinal strain compared with normal pregnancy has been demonstrated in a recent meta-analysis.15 These echocardiographic findings might explain why heart failure and secondary maternal morbidity are strongly associated with HPD.16 The incidence of heart failure with preserved LV ejection fraction (EF), defined as symptoms and signs of heart failure and evidence of LV diastolic dysfunction/raised LV filling pressures with LVEF ≥50%, during pregnancy has been increasing over the past several decades, similarly to the increasing prevalence of HDP.17 In a cross-sectional study, 17% of heart failure with preserved EF cases were diagnosed antepartum, 27% peripartum and 56% postpartum. The most significant risk factors were hypertension (CHT or HDP), anaemia, obesity, diabetes, renal disease and coronary atherosclerosis. HDP were strongly associated with the development of pregnancy-associated heart failure with preserved EF (OR for antepartum presentation 3.1; 95% CI [2.0–4.9]; OR for peripartum presentation 18.7; 95% CI [14.2–24.7]; and OR for postpartum presentation 3.3; 95% CI [2.7–4.1]).18
Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is defined as an idiopathic reduced LVEF (<45%) towards the end of pregnancy or in the first 5 months after delivery and occurs in one in 1,000 pregnancies worldwide.19 HDP, particularly PET/eclampsia, are substantial risk factors for PPCM.20 A meta-analysis of 22 studies, including 979 cases of PPCM, reported that PET was present in 22% of women with PPCM, compared with an average PET rate of 5% in the general population.20 PPCM risk might depend on HDP severity since hypertension can lead to heart failure.21 Although most women recover LVEF within 1 year after delivery, some women will have persistent cardiac dysfunction. Interestingly, in a cohort of 735 women affected by PPCM, women with PPCM and PET had more severe symptoms, signs of heart failure, a threefold greater likelihood of adverse neonatal outcomes, including termination of pregnancy, miscarriage, small for gestational age or neonatal death, but increased chances of LVEF recovery (LVEF ≥50%).22 LVEF at the time of diagnosis is the strongest predictor of long-term recovery, together with LV dilatation, right ventricular systolic dysfunction, obesity and black ethnicity.23
Even though the pathogenesis of PPCM is still unknown, researchers working with animal models have proposed molecular mechanisms, which may elucidate the relationship between PPCM and HDP. In this model, a proangiogenic vascular endothelial growth factor was strongly antagonised in late gestation by the placental secretion of soluble Fms-like tyrosine kinase 1. The increase in circulating levels of this anti-angiogenic factor, as seen in PET, correlated with the extent of cardiac dysfunction.24
Coronary Artery Disease
A population-based study of a cohort of 1,132,064 patients demonstrated that women with PET have a significantly higher risk of major adverse cardiovascular events during pregnancy, especially acute MI (AMI) and stroke.25,26 After adjusting for socioeconomic and clinical factors, women with PET had a 13-fold higher incidence of MI, an eightfold higher incidence of heart failure, a 14-fold higher incidence of stroke and a twofold higher incidence of cardiovascular-related deaths.25 A study of autopsies conducted on 34 patients with fatal eclampsia and normotensive controls showed that the former have a higher prevalence of contraction band necrosis (35%) than controls (3%).27 Contraction band necrosis, characterised by myocytes with disrupted myofibrils, is one of the earliest findings histologically seen with AMI. It is a frequent post-mortem finding in sudden deaths caused by coronary artery disease (CAD).
The most relevant obstetric risk factors for AMI in pregnancy are diabetes and HDP.28 In a cohort of 151 women, CHT, diabetes, advancing maternal age, eclampsia and severe PET were identified as independent risk factors for AMI.29 It has been estimated that in a cohort of 11 million patients, 18.3% of pregnant women with AMI experienced HDP, making PET (OR 3.2; 95% CI [2.5–4.2]) and eclampsia (OR 6.0; 95% CI [3.3–10.8]) both significant risk factors for AMI in pregnancy.30 However, as most women with AMI in pregnancy do not experience HDP, most of the risk for AMI in pregnancy is not attributable directly to HDP.26 PET and AMI share common risk factors, such as obesity, insulin resistance, CHT and dyslipidaemia, explaining the association between HDP and AMI.31,32
Cerebrovascular Disease
HDP is associated with a higher risk for ischaemic or haemorrhagic stroke during pregnancy and postpartum.33,34 Obesity, metabolic syndrome, increased inflammatory responses, hypercoagulable states and endothelial dysfunction are common risk factors for PET and stroke.35 HDP confers a higher attributable risk to maternal stroke than traditional cardiovascular risk factors, and mechanisms specific to HDP may contribute to the increased maternal risk of stroke. However, the mechanisms for the increased likelihood of cerebral injury in PET remain unknown. Studies on animal preeclamptic models have shown dramatic increases in blood–brain barrier permeability, disruption of cerebral autoregulatory function and impaired arteriolar response to neurovascular signalling.36 These changes in cerebral autoregulatory function have also been confirmed by small studies in patients affected by HDP.37 Moreover, it is generally known that PET might cause endothelial dysfunction and blood–brain barrier disruption.38 Therefore, the altered cerebral blood flow autoregulation and endothelial dysfunction associated with PET may elevate cerebral wall tension in the vessel walls and increase vulnerability to maternal stroke.38
Cardiovascular Disease in the Postpartum Period
A history of PET or GH has been recognised by the American Heart Association as a sex-specific risk factor for CVD, the leading cause of mortality in women, regardless of age and ethnicity.39,40 This recommendation highlights the fact that pregnancy might offer an ideal opportunity to screen for cardiovascular risk factors and prevent CVD later in life.
Cardiovascular Risk Factors
Most risk factors for developing PET, such as advanced maternal age, obesity and pre-existing comorbidities (such as CHT, chronic kidney disease and pregestational diabetes), are also well-recognised risk factors for cardiovascular morbidity later in life. Interestingly, it has been recently shown that women who develop PET exhibit an adverse cardiovascular risk factor profile (as determined by HbA1c, glucose and lipid profile), which deteriorates over the years leading up to the index pregnancy complicated by PET.41 In terms of established CVD risk factors arising after pregnancy, such as CHT, hypercholesterolaemia, type 2 diabetes and overweight/obesity, they explain most of the subsequent increased risk of CVD.42,43 Among cardiovascular risk factors, chronic hypertension and high BMI accounted for 77% of the excess risk of CVD in women with a history of HDP.43 Honigberg et al. showed that CHT explained 64% of HDP’s association with coronary heart disease and 49% of HDP’s association with heart failure.44 Therefore, screening for postnatal cardiovascular risk factors is crucial in managing primary cardiovascular prevention in women with a history of HDP (Figure 1).
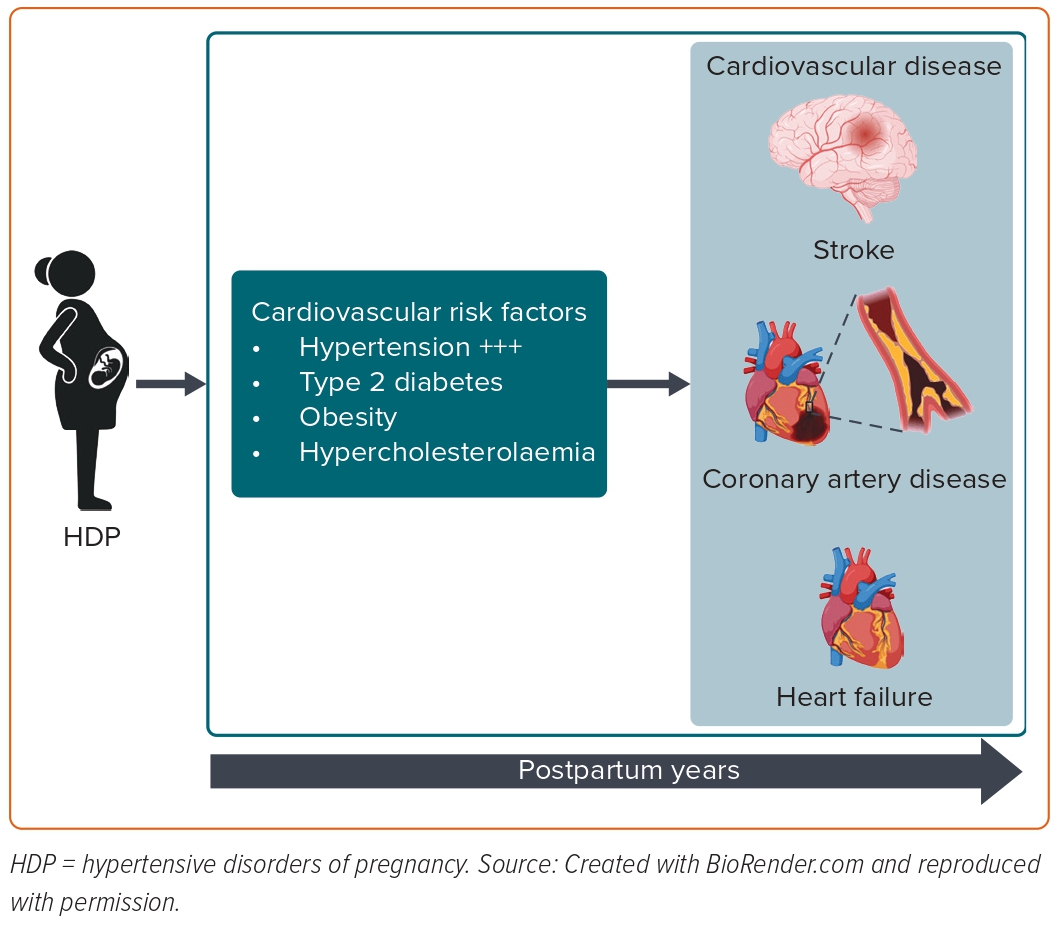
Chronic Hypertension
Women with HDP have a two- to eightfold increased risk of future CHT in the long term.45,46 Recent evidence has shown that HDP exposure dramatically increases the risk of CHT in the first months following delivery.47 The relationship between HDP, future occurrence of CHT and other prepregnancy cardiovascular risks has also been assessed to isolate the effect of pregnancy on this outcome. Most studies showed no influence from preconceptional anthropometric indices on the incidence of high BP after pregnancies complicated by HDP. Mito et al. found that women with HDP presented a higher risk of developing CHT 5 years after the index pregnancy, even after adjusting for confounding factors, such as age, BMI, family history of hypertension and salt intake (OR 7.1; 95% CI [2.0–25.6]).48 In a meta-analysis of 14 studies, after excluding women with pre-existing hypertension, the pooled OR was 5.75 (95% CI [3.92–8.44]) for the risk of persistent CHT within 2 years of delivery.47
The magnitude of the association between CHT and HDP is effectively illustrated by a Danish registry-based cohort study of 1.5 million primiparous women. It pointed out that women aged 20–29 years with a previous HDP had CHT in 10% of cases 10 years postpartum, which is comparable with the prevalence of CHT in women aged 40–49 years with previous normotensive pregnancies.49 Moreover, masked hypertension may contribute significantly to the increased cardiovascular risk in this population, as shown by a study using ambulatory and in-office BP monitoring. The authors found that 42% of women with PET with severe features, including severe hypertension, 24-hour proteinuria ≥5 g, oliguria, cerebral or visual disturbances, pulmonary oedema or cyanosis, epigastric or right upper quadrant pain, impaired liver function, thrombocytopenia, or fetal growth restriction, had persistent hypertension at 1 year postpartum, and 17.5% of these cases had masked hypertension, which was only detected by ambulatory BP monitoring.50
Several studies also examined the severity of the HDP regarding the risk of developing CHT after pregnancy.49–51 The risk of CHT also depends on the number of pregnancies affected by HDP, as was demonstrated in a meta-analysis. Women with recurrent PET had a higher chance of CHT after pregnancy than women with a subsequent uncomplicated pregnancy after PET (RR 2.3; 95% CI [1.9–2.9]).52 A multicentre randomised controlled trial showed that home BP monitoring in women with a history of PET was feasible during 1-year follow-up, and it helped increase the diagnosis of hypertension (34% in the intervention group versus 11% in the control group, p<0.001) and obtain a better BP control with 80% less hypertension at 1-year follow-up in the intervention group.53
Metabolic Syndrome and Diabetes
When a cohort of women with a history of early-onset PET were evaluated 9–16 years after the index pregnancy and compared with those with normotensive pregnancies, the former showed higher systolic BP and BMI, and more often had an abnormal lipid profile (lower HDL levels, higher triglycerides), higher glycated haemoglobin and albuminuria. They were diagnosed with metabolic syndrome in 16.8% versus 1.8% in the control group (p=0.003).54 However, the development of metabolic syndrome has been seen sooner after delivery in different studies. Metabolic syndrome has been detected in approximately 20% of PET women after 1 and 3 years postpartum. It has been estimated that the risk of developing this condition is approximately threefold higher in women with PET compared with controls.55 Large cohort studies demonstrated that the risk of developing metabolic syndrome in the immediate postpartum period was higher in early-onset PET and those PET cases with fetal growth restriction.56,57 In a Dutch study, the prevalence of metabolic syndrome in women with early PET and fetal growth restriction was >25%, which is approximately fivefold higher than the prevalence in the Dutch female population matched for age.57
A large study retrospectively studied the risk of diabetes with a median follow-up of 8.5 years postpartum, including approximately 1 million women. Women who did not develop gestational diabetes were significantly more likely to develop diabetes after GH (3.9%) or PET (6.6%) than after a normotensive pregnancy (2.5%).58 A Danish register-based cohort study also demonstrated that the adjusted risk of type 2 diabetes 14.6 years after pregnancy was 3.12 for women with GH and 3.68 for PET.59 Moreover, women with previous HDP develop diabetes earlier than women after normotensive pregnancies.60
Renal Disease
Women affected by PET and acute kidney injury (AKI) during pregnancy usually recover their pre-existing renal function postnatally. However, a prospective observational multicentre study of 237 women admitted with PET and AKI (stage 1 107, stage 2 67, stage 3 63) reported that the renal recovery rate reduced with increasing AKI stage, and that one-third of surviving women had not recovered baseline renal function by hospital discharge.61 Similarly, in a large retrospective cohort study that investigated the risk of AKI in women with a history of HDP after a mean follow-up of 6.7 years, PET was associated with AKI occurring >90 days after delivery (HR 1.22; 95% CI [1.03–1.45]).62
Chronic kidney disease, defined as a decreased renal function for ≥3 months, is an independent risk factor for CVD. Hypertensive kidney disease and glomerular/proteinuric diseases were found to be more frequent for early preterm PET (RR 3.93; 95% CI [2.90–5.33]) compared with term PET (RR 2.27; 95% CI [2.02– 2.55]). Stratifying the analyses on time since pregnancy demonstrated that associations between PET and chronic kidney diseases were much stronger within 5 years compared with >5 years after the latest pregnancy.63
The gradual decline in kidney function in patients with chronic kidney disease can lead to end-stage renal disease (ESRD), which is usually characterised by malnutrition, anorexia, nausea, vomiting, fatigue, sexual dysfunction, platelet dysfunction, pericarditis and neuropathy. A population-based study examined ESRD risk among 1.5 million women over a median follow-up time of 16.2 years (interquartile range 13.3–18.3 years). Overall, 0.15% of women with any HDP had subsequent ESRD hospitalisation compared with 0.03% of women without HDP. After adjusting for age, time and region, women with a history of PET were most at risk of ESRD (HR 4.7; 95% CI [3.6–6.0]), followed by women with a history of GH (HR 3.3; 95% CI [2.1–5.1]) compared with women with previous normotensive pregnancies.64 Women with PET in their first pregnancy had a RR for ESRD of 4.7 (95% CI [3.6–6.1]), whereas women with PET in more than one pregnancy had a RR of 15.5 (95% CI [7.8–30.8]).65
Coronary Artery Disease
In women with previous PET, the risk of CAD, including stable and unstable angina pectoris, AMI and sudden cardiac death, is increased 2.5-fold.45,46,66 Whereas after GH, the increased risk has been estimated with similar figures by three recent register-based studies, and HR ranged from 1.73 to 1.80.43,44,67 In a retrospective cohort study where 254,491 women aged 12–49 years with a live, singleton birth between 2004 and 2016 were included, both prepregnancy CHT and HDP were associated with incident CAD within 5 years of delivery (HR 3.79; 95% CI [3.09–4.65]).68 The risk of developing CAD in women who had HDP shows a ‘dose-dependent effect’ being higher in the presence of severe disease in pregnancy when associated with fetal growth restriction or iatrogenic preterm birth, and if PET recurred in subsequent pregnancies.69 The association between CAD and a history of HDP was attenuated by the presence of other cardiovascular risk factors.70 A positive history of an adverse pregnancy outcome was related to more severe disease and worse outcomes among a cohort of women with acute coronary syndrome. Women with previous PET also showed an increased risk of recurrence of acute coronary syndrome at 1-year follow-up.71
Evidence of early CAD development after PET has been increasing. Several studies have shown that the coronary artery calcium score is higher in women with previous PET than those without a hypertensive pregnancy.72 However, most of these studies were small and performed on women aged approximately 60 years. In the Cardiovascular Risk Profile: Imaging and Gender-specific Disorders study, findings from a contrast-enhanced coronary CT angiography performed in 164 asymptomatic women aged 45–55 years with a history of PET and in 387 controls matched for sex, age and race (reference group) were presented. There were signs of coronary atherosclerosis in 31% of women with a history of PET and in 18% of women from the reference group. Moreover, 47% of women with previous PET had coronary atherosclerotic plaques, and 4.3% had significant stenosis. These findings demonstrate that PET is associated with accelerated atherosclerosis; thus, early coronary atherosclerosis could precede the development of ischaemic heart disease.73
Similarly, in another recent study, 1,417 middle-aged women, including 708 women with previous PET and 709 controls, underwent CT angiography showing that the former group had a higher prevalence of any coronary atherosclerosis with an OR of 1.41 (95% CI [1.08–1.85]; p=0.012) after adjusting for age, dyslipidaemia, diabetes, smoking, BMI, menopause and parity.74 Patients with a history of PET had a significantly higher coronary artery calcium score (>0), an early marker of coronary atherosclerosis, compared with controls (16.6 versus 11.8%; p=0.009). The coronary artery calcium score is obtained using a rapid, cheap and low-radiation test. It might assess the individual risk of women with a history of HDP during their middle age, as not all HDP women have the same individual risk.75
Heart Failure
Women with previous HDP had twice the risk of CVD readmission within 3 years of delivery (6.4 versus 2.5/1,000 deliveries), and heart failure was the most common reason for readmission.76 In a retrospective cohort study of 425,649 women with any HDP, after adjustment for other risk factors, compared with women without HDP, the risk of heart failure within 5 years of delivery was increased for women with prepregnancy CHT (HR 2.55; 95% CI [1.31–4.95]), HDP (HR 4.20; 95% CI [3.66–4.81]) and prepregnancy CHT with superimposed PET (HR 5.25; 95% CI [4.24–6.50]).77
PET/eclampsia is an independent risk factor for future hospitalisations for heart failure with preserved EF, a condition with a robust female prevalence. This was demonstrated by a US retrospective cohort study of admissions for heart failure in women with (n=128,029) and without PET/eclampsia (n=240,486) after discharge from the index delivery.78 Hospitalisations due to heart failure with preserved EF were significantly more likely among women with PET/eclampsia (adjusted HR 2.09; 95% CI [1.80–2.44]), and the median time of heart failure with preserved EF onset was 32.2 months (interquartile range 0.3–65.0 months) in the entire population.78
In a Norwegian study of 508,422 women, 565 women experienced incident heart failure over 11.8 years of postpartum follow-up. GH in the first birth was not significantly associated with heart failure (HR 1.41; 95% CI [0.84–2.35]), while PET was associated with a HR of 2.00 (95% CI [1.50–2.68]). More considerable hazards of heart failure were observed in women whose only lifetime birth was complicated by PET and women with recurrent PET.79 In particular, HR for heart failure was 1.93 (95% CI [1.47–2.55]) after one single episode of PET and 4.36 (95% CI [2.71–7.02]) after recurrent PET. However, women with PET in the first pregnancy and HDP in a subsequent pregnancy had similar heart failure risk compared with women with a single lifetime pregnancy complicated by PET.79 Women with a history of HDP, including PET, GH and CHT, compared with controls demonstrated increased risks of heart failure (adjusted HR 2.11; 95% CI [1.19–3.76]) in a model adjusted for education, smoking and obesity.67 The median follow-up was 36.2 and 35.8 years for women with a history of HDP (n=571) and controls matched for age and parity (n=1,142), respectively.67
Cerebrovascular Disease
An increased risk of cerebrovascular events in women with a history of PET has been confirmed by several meta-analyses. Bellamy et al. combined four cohort studies and reported a cumulative OR of 1.81 for any stroke (95% CI [1.37–2.33]) in women with a history of PET, whereas Brown et al. reported an OR of 1.76 for cerebrovascular disease (95% CI [1.43–2.21]) for women with a history of PET. 45,46 Similarly, in a meta-analysis of 22 studies comprising >6.4 million women, including approximately 250,000 women with PET, PET was associated with a twofold increased risk of maternal stroke. The association also remained significant after adjusting for other potential confounders, including traditional cardiovascular risk factors.66 In the Framingham Heart Study, previously pre-eclamptic women had almost a fourfold higher risk of stroke within the 40 years following the index pregnancy, and in another study, the mean age at stroke onset was ≤50 years in women with HDP – both suggesting an accelerated time course to severe CVD after HDP.80
An increase in white matter lesions, independent of hypertension, has been described in women up to 5 years after pregnancies complicated by PET or eclampsia, which suggests a vulnerability to future cerebrovascular events.81 The latest data have indicated that changes in cerebrovascular structure and function are evident in those with cardiovascular risk factors, even during young adulthood.82 The presence of white matter lesions during midlife has been associated with a later risk of dementia and stroke. Therefore, it is likely that these findings in women affected by HDP might underlie their proven predisposition towards cerebrovascular accidents.
CVD-related Mortality
Because of the increased risk of CVD after HDP, CVD-related mortality has been reported to also be elevated in this cohort.45 A systematic review and meta-analysis reported in the primary analysis a pooled OR of 1.73 (95% CI [1.46–2.06]) for cardiovascular mortality after PET.83 A higher risk of cardiovascular mortality was detected in early-onset/preterm PET compared with late-onset/term PET, and in recurrent PET compared with non-recurrent PET.84,85
Interventions in the Fourth Trimester
Monitoring and treatment of postpartum hypertension are the first cardiovascular interventions that should be offered to all women with pregnancies complicated by HDP. However, high-quality evidence on the management of postpartum hypertension is lacking. The American College of Obstetricians and Gynaecologists and the National Institute of Health and Care Excellence in the UK recommend commencing antihypertensive medication at a BP threshold of 150/100 mmHg in the postpartum period, but there are no clear indications on dose reduction, cessation or management of multiple medications.86,87
A randomised controlled trial with 256 patients showed that initiating antihypertensive therapy at a lower BP threshold (140/90 mmHg) did not decrease maternal morbidity or improve outcomes, but the postnatal follow-up ended at 14 days postpartum.88 The antihypertension medications recommended in the postpartum period by the National Institute of Health and Care Excellence are calcium-channel blockers, β-blockers and angiotensin-converting enzyme inhibitors, which are considered safe during breastfeeding.86 There is no sufficient evidence to recommend a particular BP threshold or agent.89
The risk of hospital readmission because of hypertensive complications was reduced when nifedipine was used in monotherapy (compared with no treatment or with labetalol in monotherapy) or in combination with labetalol (compared with no treatment) in a large retrospective study.90 The results of the PICk-UP trial showed how the use of enalapril for 6 months after preterm PET was acceptable and feasible for patients, and it was associated with improved maternal LV parameters.91 In terms of future pregnancy, women should be informed that angiotensin-converting enzyme inhibitors are contraindicated in pregnancy. Therefore, they should consider contraception or be shifted to another antihypertensive medication preconceptionally.92,93
Another powerful tool for monitoring and, also, optimising BP control is home BP monitoring, with antihypertensive self-management to be commenced after delivery at hospital discharge. The SNAP-HT randomised controlled pilot study showed better systolic and diastolic BP control at 6 weeks postpartum, at 6 months postpartum and even at >3 years postpartum in the intervention group compared with the control group that received usual postpartum care.94 This study highlighted how the optimisation of BP level during the fourth trimester might be more important than the specific class of antihypertensive medications given postnatally.
In addition to managing hypertension, other interventions should aim to control other cardiovascular risk factors, such as obesity, metabolic syndrome, diabetes and dyslipidaemia. These interventions will include lifestyle modifications, including smoking cessation, regular exercise and diet.75,95 The risk of chronic hypertension and other cardiovascular risk factors after HDP might be markedly reduced by adherence to a beneficial lifestyle aimed at keeping a healthy weight.96 Statins should be prescribed when indicated by preventive cardiology guidelines.97 Although the risks of statins in women of childbearing potential must be considered and discussed with patients, the Food and Drug Administration removed the ‘Pregnancy Category X’ label, given emerging data on the safety of statins during pregnancy and the importance of continuing statin during pregnancy for high-risk patients, such as those with recent CAD.98
Low-dose aspirin is not routinely prescribed for primary prevention of CVD; however, it might be considered in this high-risk group aged between 40 and 70 years without increased bleeding risk.99 In a study of 83,749 women, including 4,070 (4.9%) women with a history of HDP, the risk of long-term stroke was reduced by aspirin use in the HDP group.100 Randomised trials are necessary to assess whether aspirin use could benefit women with a history of HDP in the long term.
The postpartum management of women with HDP should be based on a transdisciplinary team capable of bridging the gap between the end of obstetric care and the start of cardiovascular prevention. In addition to the cardio-obstetric team who take care of them before hospital discharge, the team should also include general practitioners, hypertension specialists and dieticians who can screen for the different cardiovascular risk factors, which can develop during the life course, and start appropriate interventions to improve the cardiovascular trajectory of women following pregnancy complicated by HDP.
Prediction of Postpartum Cardiovascular Disease
A few studies have evaluated the utility of adding information about HDP to validated calculators for cardiovascular risk.101 Women with a history of HDP, mainly PET, have an elevated Framingham risk score compared with women who experienced uncomplicated pregnancies.102 However, adding HDP to an established risk score did not improve discrimination or reclassification of cardiovascular risk.103,104 The incremental information provided by adverse pregnancy outcomes may have been partly captured by any subsequent increases in hypertension, diabetes and dyslipidaemia. Therefore, it would be more effective for primary cardiovascular prevention to identify women with HDP who will develop cardiovascular risk factors, such as CHT, diabetes and dyslipidaemia, in the first months or years postpartum, instead of CVD, which occurs several decades after HDP.
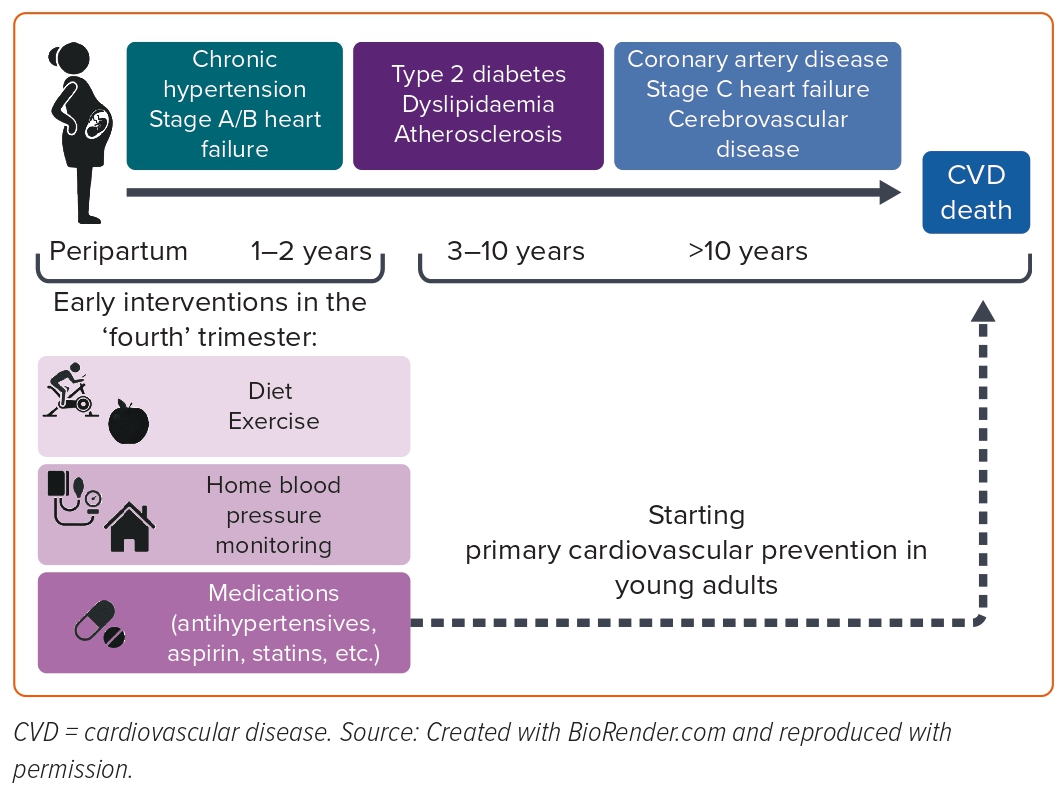
In particular, CHT is an essential target for cardiovascular prevention in women after HDP because it explains most of the excess risk of CVD in women with a history of HDP.42 Moreover, although more extensive studies are necessary to confirm these results, postpartum cardiovascular interventions, including home BP monitoring to manage antihypertensive medications and the postpartum use of enalapril, have shown substantial advantages in blood pressure control and LV remodelling, respectively (Figure 2).91,94 These peripartum interventions could be selected for women with HDP at risk of persistent CHT, and a cardiovascular screening during delivery hospitalisation might help to accomplish that.105 A prospective longitudinal study where 211 patients with HPD underwent maternal transthoracic echocardiography in the peripartum period showed that 70 out of 211 (33.2%) patients remained hypertensive at 4 months postpartum.
Regarding the peripartum characteristics, women with persistent postpartum hypertension showed worse cardiac remodelling, diastolic indices and myocardial function than women who returned normotensive. A prediction model based on clinical data (including maternal age, BMI and BP) and maternal echocardiographic indices from the peripartum demonstrated excellent discrimination in identifying women with HDP who had persistent hypertension at the postpartum follow-up.105
Conclusion
Clinicians and healthcare providers involved in caring for women with HDP should consider that women with HDP have an increased risk of acute cardiopulmonary disorders during delivery hospitalisation. Consequently, a multidisciplinary team should apply all the preventive and therapeutic measures to reduce maternal morbidity and mortality. Moreover, the increased risk of CVD in women with a history of HDP should trigger prompt patient counselling about their future cardiovascular health. Furthermore, pregnancy and adverse obstetric outcomes appear to be an incredible window of opportunity to start cardiovascular prevention. A cardiovascular screening and targeted primary cardiovascular prevention should be initiated in the peripartum period, which may be critical to risk triage and facilitate the identification of women with postpartum CHT. 










