Cardiovascular disease is the leading cause of death globally, with 85% of cardiovascular deaths attributed to acute coronary syndrome (ACS) and stroke.1 The development of coronary atherosclerosis and subsequent plaque disruption, predominantly from plaque rupture or erosion, is responsible for the majority of ACS presentations. Persistent occlusion of the coronary artery due to thrombus, leading to MI, classically presents with symptoms of chest pain and ECG evidence of ST-segment elevation.
Approximately 90% of patients with MI have angiographic evidence of obstructive coronary artery disease (CAD), based on registry studies published more than 30 years ago.2,3 The realisation that obstructive CAD was causative in the majority of patients with ST-elevation MI (STEMI) led to the development of current management strategies, including primary percutaneous coronary intervention.4 In addition to revascularisation, targeted pharmacotherapy, including high-dose statins, aspirin, P2Y12 inhibitors, beta-blockers and angiotensin-converting enzyme inhibitors, has been shown to improve outcomes in patients with STEMI in large randomised controlled trials.5–10 However, most patients in these trials had obstructive CAD.
Around 10% of patients presenting with classical signs and symptoms of ACS do not have evidence of obstructive CAD to account for their presentation, namely those with MI with non-obstructive coronary artery (MINOCA).11–13 This phenomenon has been historically overlooked and largely understudied in relation to prognosis and treatment. MINOCA was previously thought to carry a good prognosis; however, there is growing interest in this group of patients, as increasing data are showing that this syndrome is not as benign as previously thought.11,14-16 This has led to the recent authoritative paper by the European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacotherapy describing and defining the condition in detail.17
MINOCA: Definition and Terminology
To aid in appropriate evaluation, treatment and future research, the ESC Working Group on Cardiovascular Pharmacotherapy formalised the definition of MINOCA.17 The definition of MINOCA is predicated on the patient fulfilling all three main diagnostic criteria, namely: the Universal Definition of Acute MI; the presence of non-obstructive coronary artery on angiography (defined as no coronary artery stenosis ≥50%) in any potential infarct-related artery; and the absence of another specific, clinically overt cause for the acute presentation.17,18
With the Fourth Universal Definition of acute MI, the delineation of MI from myocardial injury is clearer, excluding diagnoses, such as myocarditis, where there is myocardial injury not attributable to an ischemic cause, from other causes of MINOCA.19,20 Very recently, the term troponin positive non-obstructive coronary arteries, which encompasses MINOCA, myocardial disorders and extracardiac causes, has been proposed.21 Irrespective of the nomenclature, the intention of the authors when they developed the position paper has not changed – to bring this not-so-benign condition to the attention of clinicians and to highlight the need for appropriate investigation and management. As is the case with ‘heart failure’, MINOCA is not a definitive condition, but a working diagnosis that should prompt thorough investigation to ascertain the underlying aetiology.
STEMI MINOCA versus NSTEMI MINOCA
STEMI occurs in the presence of transmural ischaemia due to transient or persistent complete occlusion of the infarct-related coronary artery. In patients presenting with non-ST-segment elevation MI (NSTEMI), the infarct is subendocardial. This pathophysiological difference also seems to be present within the MINOCA cohort. Registry data indicate that 6–11% of patients with acute MI have nonobstructive coronary arteries.11–13 Within the literature, MINOCA tends to present more commonly as NSTEMI than STEMI: the incidence of MINOCA reported in patients presenting with NSTEMI is about 8–10% and in STEMI cohorts it is 2.8–4.4%.22–25 This has resulted in an under-representation of STEMI MINOCA patients in the literature. Most studies examine undifferentiated ACS cohorts,5 with only a handful providing separate data.22–25 These studies indicate that the 1-year mortality of MINOCA presenting as STEMI is 4.5%, in contrast to the mortality of unselected MINOCA ACS patients who have a mortality of 4.7%.11,24,25 The underlying aetiology of MINOCA is similar among those presenting with STEMI and in all-comer MINOCA patients with ACS, with non-coronary aetiology responsible for presentation in 60–70% of individuals with STEMI24,25 and in 76% of unselected ACS patients.11
Clinical Features, Aetiology and Prognosis
MINOCA tends to present more commonly as NSTEMI.11,26 The clinical characteristics of patients with MINOCA are distinct from patients with conventional CAD. They tend to be younger, with a lower prevalence of hyperlipidaemia, hypertension, diabetes and smoking.11,13,27–30 As with atherosclerotic CAD, MINOCA predominantly affects men; however, the male-to-female ratio is approximately 2.5:1 with MINOCA versus 4:1 with atherosclerotic coronary disease.11
MINOCA is a heterogenous entity and consequently creates a diagnostic challenge in identifying the aetiology, which among other things may include coronary dissection, plaque rupture with embolisation, myocarditis and takotsubo syndrome. A systematic approach is required to identify the underlying cause and initiate appropriate therapy.
There have been many attempts at providing a clinical algorithm to aid physicians in evaluating and investigating these patients.17,20,31 These algorithms all underscore the same principle – namely understanding the possible mechanisms of myocardial injury, followed by investigations to determine the underlying cause. In the case of STEMI, the time for assessment prior to angiography is limited to obtaining a comprehensive history, examination and an ECG. In line with the ESC Working Group, the aetiology can be classified into three main categories: coronary, non-coronary cardiac and extra cardiac causes.
MINOCA, irrespective of the underlying aetiology, is not a benign condition. A large systematic review and meta-analysis of 1,924 patients reported that all-cause mortality in unselected patients with MINOCA at 12 months was 4.7%.11 More recently, data from the 2003–2013 SWEDEHEART registry revealed that over a mean follow-up of 4 years, 23.9% patients with MINOCA experienced another major adverse cardiac event.26 In a retrospective registry of patients presenting with STEMI, those with MINOCA were reported to have a mortality rate of 3.6% at 30 days and 4.5% at 1 year.24
Epicardial/Coronary Causes
Plaque Disruption
Atherosclerotic plaque disruption (usually plaque erosion or rupture) is the main cause of type 1 MI, which is responsible for the majority of STEMI presentations.19 However, it is also recognised that plaque disruption is not an uncommon cause of MINOCA.32–34 It is important to differentiate patients with ≥50% epicardial coronary artery stenosis from those without obstructive CAD. Furthermore, positive remodelling of the coronary arteries may result in compensatory enlargement of the culprit vessel, increasing the luminal size of atherosclerotic arteries. This may consequently result in the appearance of angiographically normal or minimally obstructed coronary arteries.35 Plaque disruption has been documented in patients with angiographically near-normal arteries, namely those with luminal irregularities.33,34 Therefore, it is possible that patients with mild luminal irregularities presenting with STEMI have underlying atherosclerosis with plaque disruption, thrombosis and transient occlusion from distal embolisation. In the presence of effective endogenous thrombolysis, such individuals may not exhibit angiographic evidence of significant plaque or visible thrombus.36
Appreciation of the importance of plaque rupture as the underlying pathomechanism in patients presenting with MINOCA has been facilitated with the use of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) imaging. Advanced intracoronary imaging is frequently required to diagnose plaque disruption. Such techniques have shown evidence of plaque rupture in 37% of patients with ACS who had angiographically unobstructed coronary arteries.32 A small study combining IVUS and cardiac MRI has shown that the majority of patients with MINOCA with plaque disruption have cardiac MRI evidence of acute myocardial oedema, providing the much needed link between areas of plaque disruption and ensuing myocardial injury.33 It is estimated that one-third of MINOCA presentations are attributable to plaque disruption, although the proportion among those presenting with STEMI is less clear because they are under-represented in the studies (13–39% of subjects) and advanced imaging techniques are less often used in these scenarios.24,32,33
Coronary Dissection
Spontaneous coronary artery dissection (SCAD) describes the acute spontaneous development of a false lumen within the coronary artery, compromising flow down the artery. This condition has a strong female preponderance, with a mean age of 44–53 years, and is associated with fibromuscular dysplasia and pregnancy, indicating that female sex hormones may play a role in its pathophysiology.37 The reported incidence of SCAD among patients presenting with ACS is between 2% and 4%.38,39
Diagnosis of SCAD is made during angiography, where it can be subclassified based on angiographic appearance.40 It is recommended that coronary instrumentation – including stenting – is avoided, where possible, particularly if epicardial coronary flow is normal.37 In cases where there is diagnostic uncertainty or where coronary intervention is required, intracoronary imaging with OCT or IVUS can be useful to make a definitive diagnosis and also to assess the outcome of intervention.
On initial angiography, SCAD, in particular type 2 SCAD, can be easily missed. Careful review of fluoroscopy images, especially in patients who exhibit high-risk demographics (typically middle-aged peripartum women without traditional cardiac risk factors) or those who have high-risk clinical features (e.g. history of fibromuscular dysplasia, connective tissue disorder, recent intensive exercise or emotional stress) could allow a diagnosis to be made and appropriate treatment to be initiated.40
Coronary Vasospasm
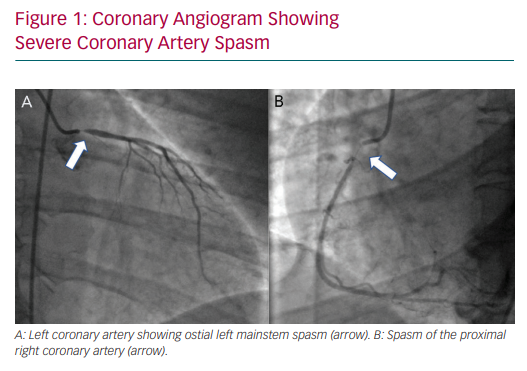
In 2015, the Coronary Vasomotion Disorders International Study Group defined the diagnostic criteria for vasospastic angina as: nitrate-responsive angina, transient ischaemic ECG changes and coronary artery spasm (Figure 1), defined as transient total or subtotal (>90%) occlusion either spontaneously or in response to a provocative stimulus.41 Vasospasm, like SCAD, is more prevalent in women. Risk factors include smoking and the use of drugs such as cocaine or beta-blockers in patients with vascular smooth muscle hyperreactivity.42–46
Its transient nature and responsiveness to nitrates makes the diagnosis of coronary vasospasm in STEMI a challenge, as the frequent use of intra-arterial isosorbide mononitrate administration during radial cannulation to prevent peripheral vasospasm could essentially mask the diagnosis. For this reason, the use of a radial cocktail is prohibited during provocative vasospasm testing.42
The gold standard diagnostic test for coronary vasospasm is provocative spasm testing with the administration of a spasm-provoking stimulus, namely acetylcholine or ergonovine. The patient is required to experience chest pain and demonstrate ECG and angiographic changes in response to provocation before the test is deemed to be positive for the diagnosis of spasm.41 Invasive testing is relatively safe, with no irreversible complications in patients with a recent ACS, and therefore should be considered as part of the investigative workup of STEMI patients with MINOCA.47,48 Clearly it should be done outside of the acute presentation.
Coronary Thromboembolism
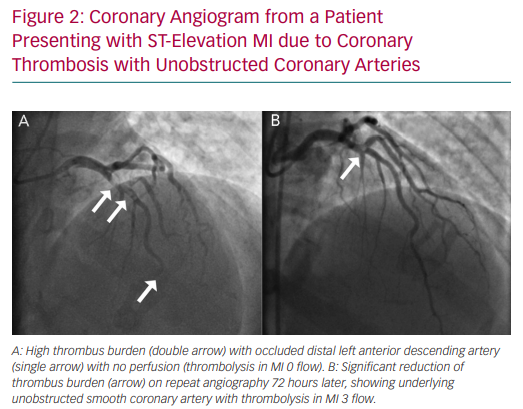
Coronary embolism is the underlying cause in 3% of ACS presentations, but is often under-recognised because it is hard to differentiate from atherosclerotic ACS.49 Coronary thromboembolism can arise from either the left atrium or by means of paradoxical embolisation in the presence of septal defects in patients with hypercoagulable states, such as those with AF or those with hereditary thrombophilias. Non-thrombotic emboli can arise from valvular vegetations or cardiac tumours.50 In patients who exhibit a high thrombus burden at angiography without underlying coronary atherosclerosis, thromboembolism or thrombophilia should be strongly suspected (Figure 2).49
Unlike SCAD and coronary vasospasm, patients with coronary thromboembolism are more heterogenous and can have a wide variety of predisposing clinical characteristics. The most frequent predisposing condition is AF, followed by valvular heart disease, such as infective endocarditis or rheumatic heart disease.51
In one of the largest systematic reviews of patients with MINOCA, up to 14% of patients were reported to have had evidence of an inherited thrombotic disorder.11 In a more recent study, extensive thrombophilia testing revealed that 33% of MINOCA patients had inherited thrombophilia.52 These findings imply that coronary embolism might have previously been overlooked as the underlying cause of MINOCA in this cohort. The diagnosis of a thrombotic disorder as a cause of STEMI will change the management of these patients, usually mandating lifelong anticoagulation rather than the standard dual antiplatelet regimen prescribed for ACS.
Non-coronary Cardiac Causes
Takotsubo Cardiomyopathy
Takotsubo or stress cardiomyopathy describes the transient impairment of left ventricular function (commonly sparing the basal myocardium), frequently precipitated by a stressful event.53 It typically occurs in postmenopausal women, most often (but not exclusively) with a precipitating acute emotional or physical stressor.53 For this reason, it has been thought to be a catecholamine-driven process. Approximately 2% of STEMI cases are considered attributable to takotsubo cardiomyopathy.54
Patients generally present with symptoms of chest pain and ECG changes consistent with ACS, together with troponin elevation. The troponin level is usually minimal in relation to the extent of N-terminal prohormone brain natriuretic peptide (NT-proBNP) elevation, demonstrating a mismatch between the markers of myocardial ischaemia and the extent of myocardial involvement.55,56
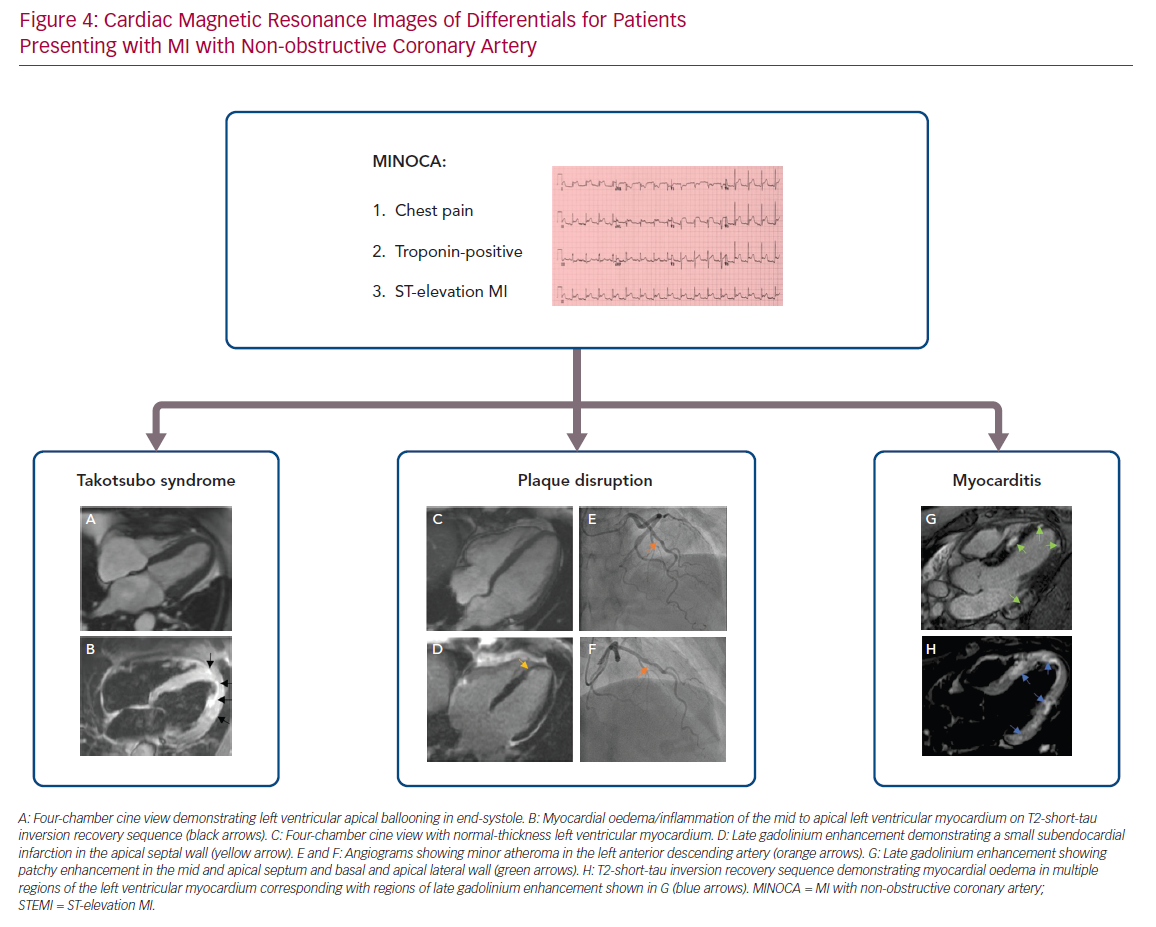
The Heart Failure Association of the ESC has defined the diagnostic criteria for takotsubo cardiomyopathy, which takes into consideration biomarkers and imaging. Patients should have transient regional wall motion abnormalities of the myocardium that recover on follow-up imaging, extending beyond a single epicardial coronary distribution with absence of coronary culprit lesions, in the context of new ECG changes, significantly raised NT-proBNP or BNP and a relatively small rise in troponin.56 The recommended first-line investigation is a transthoracic echocardiogram, as it can be used to assess the anatomical variant, complications and recovery. Cardiac MRI imaging, if performed early after the event, can accurately characterise regional wall motion abnormalities, myocardial oedema and patterns of injury with late gadolinium enhancement in patients with takotsubo syndrome. Furthermore, cardiac MRI allows differentiation from other causes, including myocarditis and infarction.56 Therefore, early assessment with biomarkers, echocardiography and, if available, cardiac MRI is recommended to determine and confirm the diagnosis.57,58
Myocarditis
Inflammation of the myocardium secondary to a variety of infectious pathogens, autoimmune conditions or toxins may imitate an ACS. Individuals may present with symptoms of chest pain, ST-segment elevation, raised troponin level and impaired left ventricular systolic function.59 Owing to the wide spectrum of aetiology, the clinical characteristics of patients with myocarditis vary widely; therefore, making the correct diagnosis requires a high index of suspicion during presentation.
The gold standard test for diagnosing and determining the aetiology of myocarditis is endomyocardial biopsy,59 although this is associated with a risk of complications – particularly in young individuals – and should be reserved for patients with haemodynamic compromise, severely impaired left ventricular function that is unresponsive to treatment and/or significant ventricular arrhythmias.60 In such individuals, early cardiac MRI can prove valuable in the diagnosis of myocarditis, with sequences for tissue characterisation.60–62 In cases of MINOCA where there is suspicion of myocarditis, cardiac MRI during acute admission can be extremely helpful in confirming or refuting the diagnosis.62,63
Extracardiac Causes
Type 2 MI
A mismatch between oxygen supply and demand, leading to ischaemic myocardial injury, is responsible for type 2 MI and should be differentiated from conventional atherosclerotic plaque disruption.19 Patients with type 2 MI tend to be older and have several comorbidities when compared to those with type 1 MI.64 Common causes of increased oxygen demand from cardiac myocytes include sustained tachyarrhythmias or reduced oxygen supply, for example in acute bleeding conditions or pulmonary embolism, where patients with otherwise mild or stable CAD could develop myocardial ischaemia. However, such a mismatch would have to be profound to cause MINOCA.
Aortic Syndromes
Acute aortic dissection results from a tear within the wall of the aorta forming an intimal flap separating the false and true lumens. Classification is based upon the location and extent of the dissection. The incidence is higher in men and increases with age.65 Risk factors include uncontrolled hypertension, pre-existing aortic disease or connective tissue disorders, blunt chest trauma and intravenous drug abuse.65 Similar to ACS, patients commonly present with acute severe chest pain; however, the nature of the pain is dissimilar, usually manifesting as a sharp, tearing or ripping sensation.65
Only about 18% of aortic dissection presents with MI, particularly type A dissection.66 Coronary artery involvement may occur due to the flap occluding the coronary ostium or as a result of extension of the dissection into the coronary artery. Early surgical intervention is recommended to improve survival, given the high mortality rate without intervention.65 In patients with MINOCA, the use of direct aortography may provide the diagnosis, although non-invasive investigations, such as echocardiography or CT aortography, are safer and recommended as first line.65
Assessment
Initial Investigations
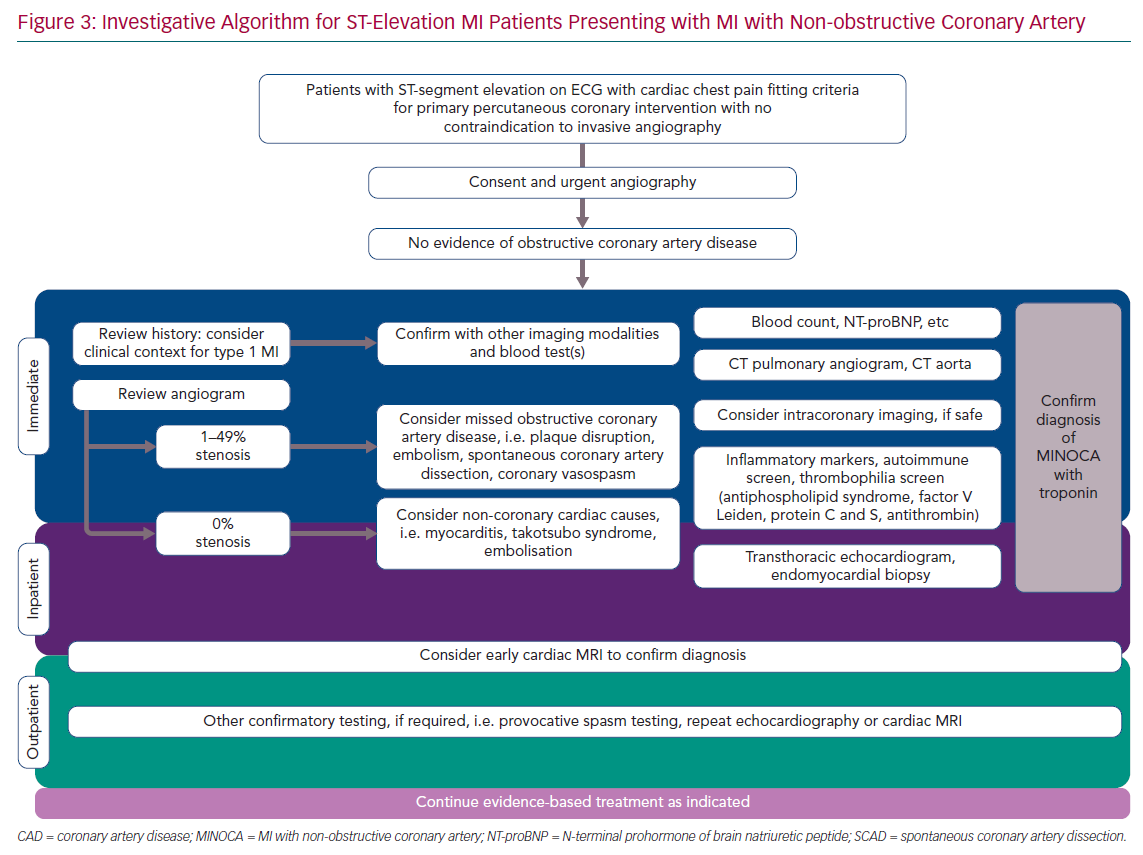
As part of the investigative process, it is recommended that all patients who present with acute ST-elevation have emergency assessment of their clinical history with essential examination, an ECG or serial ECGs confirming ST-elevation or new-onset left bundle branch block, and coronary angiography with the option of proceeding to coronary angioplasty.4 Baseline blood tests should be taken before angiography, but cardiac catheterisation should not be deferred until the blood results become available. Following angiography, in the case of the patient without obstructive coronary disease, re-evaluation of the patient’s clinical history could prove valuable in helping identify the underlying diagnosis (Figure 3).
Thorough review of the fluoroscopic images obtained during initial coronary angiography may reveal otherwise subtle changes. If there are any doubts, and provided it is safe to do so, further intracoronary imaging may be useful to confirm or refute differential diagnoses. Given the high incidence of plaque disruption documented with intravascular imaging in patients with MINOCA, the use of OCT or IVUS of atherosclerotic non-obstructive diseased vessels could prove useful in identifying the underlying pathology in patients with STEMI.32–34
In patients with a large thrombus burden and normal coronary arteries, thromboembolism should be considered and thrombophilia screening performed as part of diagnostic workup. Consideration of different thrombophilic disorders should prompt extensive testing for inherited thrombophilia. This includes testing for factor V Leiden, antiphospholipid syndrome, prothrombin G20210A mutation, proteins C and S and antithrombin III deficiency.52
Plaque disruption is extremely unlikely in patients with smooth, unobstructed coronaries without evidence of atherosclerotic disease at angiography, as shown in several studies involving intracoronary imaging.32–34 Therefore, clear distinction of patients according to degrees of coronary stenosis (i.e. 0% compared to 1–49%) may prove invaluable in identifying the underlying aetiology.
During initial coronary angiography, the presence of coronary spasm helps clarify this diagnosis in patients presenting with STEMI and suspected MINOCA; however, occasionally provocative spasm testing may be required at a later stage to fulfil the aforementioned diagnostic criteria as a class I indication in patients with MINOCA.41 There are no data to support provocative testing in patients with STEMI and suspected MINOCA at the time of initial angiography. However, recent studies appear to show that provocative spasm testing is safe in ACS patients, with reversible complication rates comparable to diagnostic angiography (a serious adverse event rate of around 0.8%).67,68 Small studies demonstrate that during the acute phase there are no irreversible complications; bradyarrhythmias occur in 5–16% of cases, which is comparable to that seen in stable patients with non-obstructive coronary arteries (15%),and ventricular tachyarrhythmias are rare (<0.5%), but this is clearly not in the setting of STEMI.48,69 There are important prognostic benefits to reaching a correct diagnosis and accurately identifying and treating patients with coronary vasospasm, therefore routine vasospasm provocation testing is strongly encouraged in patients with MINOCA.48,70
Follow-up Investigations
Following cardiac catherisation in patients with a provisional diagnosis of MINOCA, other blood investigations to consider include a full blood count (checking for the presence of significant anaemia in the case of a type 2 MI), inflammatory markers and a thrombophilia screen. A detailed transthoracic echocardiogram can help elucidate potential features of myocarditis or valvular disease. Early cardiac MRI within the first 5–14 days of admission is recommended, where possible, to confirm a diagnosis of MI or other aetiologies of MINOCA (Figure 4).71–73 A recent study has shown that cardiac MRI can offer a definitive diagnosis in 88% of patients with MINOCA and, more importantly, changed the diagnosis in 47% of cases.74
Treatment
In contrast to the definite aetiology and guidelines for the management of STEMI with CAD, in the 10% of patients who experience MI in the absence of obstructive CAD the aetiology often remains unclear, mainly because it is under-investigated and the optimal management is therefore often undecided.14
There are no prospective randomised trials of pharmacotherapies in patients with MINOCA. Retrospective data from the SWEDEHEART registry suggest that there is long-term prognostic benefit in treating such patients with statins, beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, irrespective of the underlying aetiology.26 However, there appeared to be no significant benefit associated with the use of P2Y12 inhibitors. Thus, it appears that the use of routine secondary prevention medications post-ACS can still prove beneficial in MINOCA, despite a possibly unclear aetiology.
The obvious limitation is that SWEDEHEART is a retrospective observational study where many confounders are present that are not accounted for. Furthermore, the majority of MINOCA presentations are attributable to plaque disruption and myocarditis,which may benefit from conventional secondary prevention pharmacotherapy, especially in the context of left ventricular systolic dysfunction.11,20,24
The use of dual antiplatelet therapy (DAPT) is more controversial, since in the SWEDEHEART registry the treatment of patients with DAPT was not shown to confer benefit.26 Furthermore, in the post-hoc analysis of the Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events – Seventh Organization to Assess Strategies in Ischemic Syndromes (CURRENT-OASIS 7) study evaluating the use of DAPT in MINOCA and non-MINOCA patients, using high-dose DAPT with double-dose clopidogrel increased the risk of major adverse cardiovascular events (HR 3.57; p=0.013) without an increase in bleeding.75 With the limitations of being a post-hoc analysis with multiple confounders, this poses an interesting hypothesis that DAPT may actually be harmful when used in MINOCA patients.
Current and Future Studies
A number of studies are in the pipeline to investigate the optimal assessment and treatment options for patients with MINOCA. The Women’s Heart Attack Research Program – Imaging Study (HARP; NCT02905357) is a multicentre, prospective, observational study aiming to recruit 500 women with MINOCA who will undergo OCT at the time of diagnostic angiography and cardiac MRI to explore the proportion of plaque disruption and its correlation with cardiac MRI findings. Recruitment started in 2016 and the study has an estimated completion date of April 2020. The multicentre Randomized Evaluation of Beta Blocker and ACEI/ARB Treatment in MINOCA Patients (MINOCA-BAT; NCT03686695) trial is exploring the impact of beta-blockers and angiotensin-converting enzyme inhibitor or angiotensin receptor blockers on the composite endpoint of death from any cause and readmission due to acute MI, ischaemic stroke or heart failure. It is aiming to recruit 3,500 patients. Enrolment started in 2018 and the trial has an estimated completion date of 2025.
Conclusion
MINOCA is a condition with comparable in-hospital and long-term mortality to conventional ACS. The heterogeneity in aetiology often makes it a challenge for clinicians to investigate and treat this condition optimally. In STEMI patients with MINOCA, careful assessment of the history and examination findings during initial contact, with meticulous review of the coronary angiogram, can allow appropriate triaging. Routine biomarkers, including troponins and NT-proBNP, and early use of cardiac MRI imaging is advocated. Additional selective use of further testing that includes intracoronary assessment with IVUS/OCT, thrombophilia screening and vasoprovocation testing should be considered on an individual basis. Unless alternative causes are identified that mandate specific treatment, patients presenting with MINOCA should receive standard post-ACS pharmacotherapy.
Ongoing studies will hopefully yield new insight into the optimal management of this hitherto under-investigated condition.