Cardiovascular and neoplastic diseases are currently the main causes of mortality and morbidity in developed nations.1 These diseases often coexist in the same individual, worsening general condition, complicating therapeutic management and – from a different perspective – raising the issue of possible shared pathophysiological pathways between cardiovascular disease (CVD) and cancer. Many of the known risk factors for the development of CVD also increase the risk of cancer.2,3 In this complex setting, the debate about the oncogenic potential of some classes of cardiovascular drugs has recently come to the fore. In 2017, three important events focused attention on the postulated oncogenic potential of some antihypertensive drugs. First, news about the US Food and Drug Administration’s withdrawal of some batches of very popular and widely used antihypertensive drugs, such as losartan, valsartan and irbesartan, due to the presence of potentially carcinogenic byproducts of the pharmaceutical process (N-nitrosodimethylamine, N-nitrosodiethylamine and N-nitroso-N-methyl-4-aminobutyric acid).4 Second, the publication of two Scandinavian trials suggesting that thiazide diuretics may play a role in the development of skin neoplasms;5,6 and third, one trial claiming that angiotensin-converting enzyme (ACE) inhibitors may cause lung cancer. These events generated wide concern among physicians and patients, monopolising the medical pages of newspapers and magazines as well as doctor–patient interactions in offices and hospitals. They are part of a debate born in the first half of the 1900s, enriched over time by data obtained mainly from national registers and cohort studies, and only exceptionally by randomised controlled trials (RCTs) which have evaluated the carcinogenic potential of specific antihypertensive drugs. This disparate data collection has jeopardised the reliability of evidence in this setting. In fact, over time, different dosages and associations of the active principles may have varied and other concomitant risk factors for cancer may have intervened/disappeared without leaving a trace in the records of observational studies or registers.7 In post hoc analysis, preferential recall can occur and adherence to therapy (which is crucial for chronic therapy) is not usually assessed.8 Furthermore, concomitant or previous therapies that were not recorded may have contributed to the modified risk of cancer.6 In general, observational trials lack randomisation, whereas post-hoc meta-analyses of RCTs may include important differences in control populations: normotensive patients, non-pharmacologically treated hypertensives and hypertensive patients treated with different active treatments.9,10 These differences make it very difficult to use the data generated to establish firm conclusions about the risk of cancer in relation to antihypertensive use.
In light of recent events, this article provides a critical overview of the evidence supporting the carcinogenic role of diuretics, angiotensin receptor blockers (ARBs) and ACE inhibitors in specific settings. Data for this review were identified by a PubMed search going back to 2000 and by analysing articles mentioned in the references of papers yielded by the search. Search terms concentrated upon the prevalence of cancer and selected antihypertensive drugs (diuretics, ACE inhibitors and ARBs). Representative studies for various drugs and cancer in patients with hypertension were included, giving priority to meta-analyses or studies with larger sample sizes.
Antihypertensive Drugs and Cancer Risk
Diuretics
Renal Cancer
Diuretics are largely used as antihypertensive medications, often in combination with other therapies, therefore it may be difficult to assess the net adverse effect of a single diuretic. In addition, diuretics have been often investigated as one single class. Nonetheless, thiazides have been investigated more closely since the 1980s.11,12 The potential carcinogenic role of thiazides appears to be supported, at least with regards to kidney cancer, by their toxic and mutagenic effect at the distal tubule level during long exposure to these drugs.13,14 Moreover, the toxic metabolites of thiazides and loop diuretics, namely N-nitroso derivates, have been accused of having tumourigenic actions.15
With regards to renal cancer, available data often come from observational studies or retrospective analyses with incomplete assessment of renal cancer risk factors and different cut-offs or means (self-reported or office-based readings) to diagnose hypertension.16 Despite this, most cohort studies and population studies in humans have shown that diuretics, in a dose-dependent fashion, may contribute to a twofold to fourfold increase in the risk of developing renal cell carcinoma, particularly in women.17,18 Recently, a case control study that failed to find any association between antihypertensive drugs and renal carcinoma overall found a positive association between both diuretic and calcium-channel blocker use and papillary renal carcinoma, even in a limited number of hypertensive people.19 In contrast, no significant association has ever been found between diuretic use and renal cell cancer in normotensive people.20–22 Therefore, whether the presence of hypertension itself might be a risk factor for renal cell carcinoma has long been questioned.18
A recent meta-analysis considering evidence from 85 prospective studies found a positive association between hypertension and kidney cancer.23 This evidence seems to be supported by the fact that many metabolic and neuro-hormonal pathways, such as the renin–angiotensin–aldosterone system (RAS), catecholamines and vasopressin, may play a role in both cancer development and hypertension.24–26 The most recent evidence in this setting comes from a systematic review of 27 observational studies that found an association between diuretic use and the risk of kidney cancer (RR 1.34, 95% CI [1.19–1.51]) that increased with the duration of treatment and was still significant after adjusting for hypertension and smoking.27
Skin Cancer
A possible association between diuretic use and skin cancer was hypothesised a long time ago. This suggestion has recently become more important because of the increasing incidence of cutaneous melanoma and non-melanoma skin cancers.28 The main risk factors for skin cancer development are exposure to ultraviolet light, fair skin, light eye and hair colour, the presence and number of common and atypical naevi and a tendency to develop freckles. These factors have not been assessed in most available trials. In addition to this, the time between exposure to diuretics and skin cancer development appears to be too short to be biologically plausible in most studies; therefore, data should be interpreted with caution.7
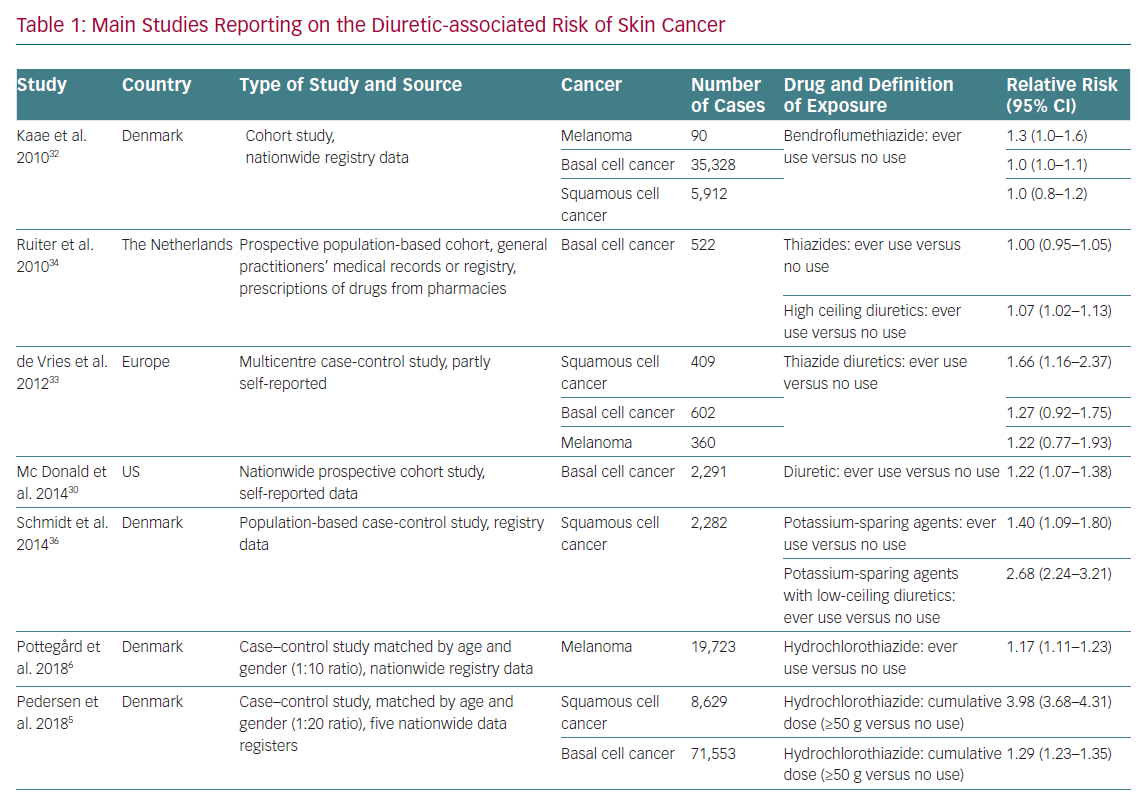
The association between diuretics, in particular thiazides, and skin cancer is supported by the fact that they are photosensitisers, causing direct damage to DNA and chronic subclinical skin inflammation. However, photosensitivity is also a common reaction to many widely used drugs, such as antibiotics, non-steroidal anti-inflammatory drugs and statins, which are often used in the polypharmacy of hypertensive patients.29,30 Self-reported consumption of diuretics, including thiazides, was associated with an increased risk of basal cell carcinoma (BCC) in a nationwide US population-based cohort study, but other studies have not reported similar results (Table 1).31–33 De Vries et al. observed an association between bendroflumethiazide and BCC, but this finding was no longer significant after correction for multiple hypothesis testing.34 Information reported on specific diuretics found an increased risk of BCC among users of loop diuretics, especially long-term users.35
A 2008 observational study found an increased risk of squamous cell carcinoma (SCC) and malignant melanoma in users of amiloride alone or in combination with hydrochlorothiazide, and of malignant melanoma in users of sulphonamides and other low-ceiling diuretics (e.g. indapamide).36 In 2015, a subsequent case control study including data from this cohort plus data from population-based databases in northern Denmark during 1991–2010 showed that long-term diuretic use was associated with an increased risk of SCC, driven by potassium-sparing agents alone or in combination with low-ceiling diuretics.37 The recent north European nationwide case control study by Pedersen et al. found a clear dose–response relationship between hydrochlorothiazide use and both BCC and SCC, especially the latter cancer type.5 In this analysis, the use of high cumulative doses of hydrochlorothiazide (>50 g) was associated with a dose-dependent increase in the risk of BCC (OR 1.29, 95% CI [1.23–1.35]) and SCC (OR 3.84, 95% CI [3.68–4.31]). The proportion of skin cancers attributable to hydrochlorothiazide use was 0.6% for BCC and 9.0% for SCC. The risk was higher in women than men and in patients <50 years old. This increased risk was not observed with chlorthalidone or indapamide. The main limit of these north European trials is the lack of information on two major risk factors for BCC and SCC, namely ultraviolet exposure and skin phenotype.
A recent meta-analysis of 19 studies from 1993 to 2016 concerning diuretic use and the risk of skin cancer found a non-significant 30% increase in skin cancer risk among thiazide users (standardised RR 1.31, 95% CI [0.93–1.83]), based on 11 RR estimates from six independent studies.38 This meta-analysis excluded trials involving different active arms, normotensive people as controls and those lacking distinction between different diuretics. The latest meta-analysis comprising all available studies examining the risk of developing skin cancer with thiazide use found an association between chronic exposure to thiazides and all skin cancers, particularly between the use of hydrochlorothiazide – alone or in combination – and the risk of developing SCC.39 It must be pointed out that this analysis is based on few and generally heterogeneous observational studies for every type of skin cancer and many of these studies do not consider the interaction between different skin types, sun exposure and family history of skin cancer. Despite this, the association between thiazide diuretics and SCC risk appears to be supported by the epidemiological evidence available to date.
With regards to melanoma skin cancer, a recent meta-analysis found a RR of 1.17 (95% CI [1.12–1.24]) for the association between thiazide diuretic use (ever versus never) and melanoma risk based on four independent RR estimates. The heterogeneity of studies was low, but the results were heavily influenced by the Danish study by Pottegård et al. that found an increase in melanomas, specifically nodular and lentigo melanomas, in patients treated with hydrochlorothiazide.6,40,41
Based on recent evidence, the British and Irish Hypertension Society recommended that thiazide-like drugs (e.g. chlorthalidone or indapamide) be preferred over thiazide diuretics (e.g. hydrochlorothiazide or bendroflumethiazide) when starting hypertension treatment but should not be discontinued in patients with well-controlled blood pressure levels.42 Likewise, the European Medicines Agency has stated that hypertensive patients treated with hydrochlorothiazide should be informed about the risks, limit their exposure to ultraviolet rays and regularly check their skin, but should not stop treatment unless they have a history of non-melanoma or melanoma skin cancer.43
Renin–Angiotensin–Aldosterone System Blockers
Until recently ARBs were prescribed for several indications beside hypertension with no major safety concerns, but in the past 2 years the US Food and Drug Administration has issued more than 1,000 communications regarding the withdrawal of batches of drugs containing valsartan, irbesartan and losartan, often in fixed-combination pills, due to the presence of excessive quantities of nitrogen derivatives known to have oncogenic effects.4 These impurities were added during the processing of active principles at specific sites in China and India. However, these measures have contributed to the identification of ARBs as oncogenic themselves.
From a pathophysiological point of view, the role of angiotensin and its receptors in the development neoplasms appears to be complex. Many types of cancer express type I angiotensin II receptors, suggesting that angiotensin may play a role in mediating processes such as progression, vascularisation and metastasis; therefore, the use of ACE inhibitors and ARBs could be protective from an oncologic point of view.44–46 The proposed mechanisms mediating the anti-tumour activity of ACE inhibitors and ARBs include, among others, the inhibition of matrix metalloproteases and reduced expression of vascular endothelial growth factor, but the role of the consequent unopposed chronic overstimulation of the type II angiotensin receptors is uncertain, as is the increase in renin levels resulting from the blockade of type I angiotensin II receptors.47–49 On the other hand, the increase in angiotensin (I–VII) occurring during ARB treatment might have an anti-angiogenic effect in tumours.50
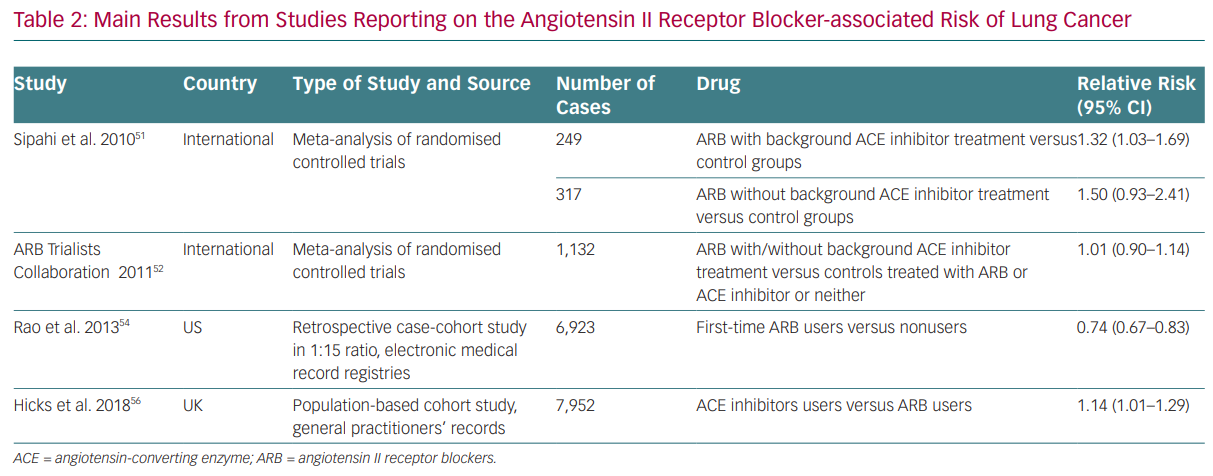
Whereas most of the evidence on ACE inhibitors and ARBs is neutral on cancer risk, some data have been reported since the beginning of RAS modulation therapy that suggest the risk of cancer cannot be ruled out (Table 2). For instance, results from the Candesartan in Heart Failure Assessment of Reduction on Mortality and Morbidity (CHARM) study first pointed towards the possible link between RAS inhibition and cancer development.51 It reported a significantly increased risk of cancer in patients exposed to candesartan (2.3%) compared with placebo (1.6%).
With regards to cancer development and cancer-related mortality, in 2011 Sipahi et al. published a meta-analysis based on the evaluation of studies with ARBs reporting cancer data, suggesting that ARBs were associated with a ‘modest increase’ (1–2% absolute risk) in new cancers, especially lung and prostate cancers, as compared with the control group (7.2% versus 6.0%; RR 1.08, 95% CI [1.01–1.15]).52 This meta-analysis included studies with a follow-up of 1 year, the longest follow-up being 5 years, which seems too short to draw conclusions on cancer development. In addition, there was no unique assessment of cancer diagnosis in the studies, data were lacking about other risk factors (such as previous history of cancer) and there were different comparator agents in the meta-analysis. The results were mainly driven by the Renal Outcomes with Telmisartan, Ramipril, or both, in People at High Vascular Risk (ONTARGET) study, which studied telmisartan in association with ramipril. In the same year, the ARB Trialists collaborators published an analysis of individual data from 15 multicentre
double-blind clinical trials involving more than 130,000 individuals at high cardiovascular risk assigned to telmisartan, irbesartan, valsartan, candesartan or losartan, with or without an ACE inhibitor.53 The study included 75,000 more individuals than the meta-analysis by Sipahi et al. and had different control groups (ACE inhibitor, calcium-channel blocker or placebo). It found no increase in overall or site-specific cancer risk from individual ARBs when compared to control.53 Similarly, a huge meta-analysis of more than 300,000 subjects excluded any association between ACE inhibitors and ARBs and an increased risk of any cancer or cancer-related mortality. However, this study was unable to rule out an increased risk of cancer with the combination of ACE inhibitors and ARBs.54 Subsequently, a US nationwide retrospective observational study relying on an administrative database and involving around 70,000 cases and more than 1 million controls, with a follow-up of around 5 years, excluded any association between lung cancer and ARB therapy.55 On the contrary, ARB use appeared to have a protective effect (HR 0.74 [0.67–0.83], p<0.0001), with a small absolute risk reduction of 0.30 lung cancers per 1,000 person-years in the ARB-treated group.
Finally, a recent meta-analysis of 19 RCTs with at least 12 months of follow-up data reporting on cancer incidence involving about 140,000 patients found no significant differences when comparing an ARB with placebo, an ARB with an ACE inhibitor, an ARB plus partial use of ACE inhibition with placebo plus partial use of ACE inhibition or ARB in combination with an ACE inhibitor.56 Data from the Valsartan in a Japanese population with hypertension and other cardiovascular diseases (Jikei Heart Study) and KYOTO HEART studies included in the meta-analysis by Bangalore et al. had been retracted from publications by this time due to unreliable data and were thus excluded from this analysis.
ACE inhibitor users were found to have a 14% greater risk of developing lung cancer than ARB users by a cohort study of more than 900,000 patients who started receiving treatment between 1995 and 2015 and who were followed up for 6.4 years.57 The risk increased with longer duration of use and was significant after 5 years. The authors claimed that bradykinin and substance P, whose levels increase during ACE inhibitor therapy, had a supporting role in tumour proliferation. These data have been deeply criticised. Indeed, the authors compared ACE inhibitors to ARBs, which may have a role in cancer promotion/inhibition. Moreover, smoking status did not appear to change the risk of cancer in the ACE inhibitor group and the authors failed to prove an association between ARB-based therapy and cancer in a subsequent analysis of non-smokers, which is highly difficult to explain. In addition, smoking duration and intensity were not completely assessed in all populations. Moreover, persistent cough is a common side effect of ACE inhibitors, raising the possibility that their observed association with lung cancer could be due to detection bias; patients taking ACE inhibitors may be more likely to undergo diagnostic evaluations, leading to increased detection of preclinical lung cancers. Despite this, in our opinion, hard evidence is still lacking in this setting.
Conclusion
The possible carcinogenic roles of drugs, foods and lifestyles has been the focus of much scientific interest over the past 50 years. The possibility that some drugs, which have dramatically modified life expectancy and quality of life by contributing to the control of CVD, may increase the risk of cancer when taken over the long term is unquestionably alarming. For this reason, some recently published data – even if not completely free from limitations – have contributed to discussion in this area. This overview of data on the role of diuretics, ARBs and ACE inhibitors in promoting the development of neoplasms highlights the considerable difficulty of deriving reliable evidence in this setting. Although RCTs are ethically unfeasible, observational studies usually present biases that limit their reliability. For this reason, ‘big data’ need to be collected in a more in-depth manner to produce evidence-based recommendations in the future. At this time, recommendations to interrupt successful antihypertensive therapies with ARBs, ACE inhibitors or diuretics to avoid a generic risk of cancer do not appear to be justified; watchful waiting and paying attention to patients whose cancer risk appears to be increased seems the best strategy.