The ISCHEMIA and REVIVED-BCIS2 trials have highlighted that percutaneous coronary intervention (PCI) in patients with stable coronary syndrome provides limited additional prognostic benefits when used on top of optimal medical therapy.1,2 This has led to much attention being paid to coronary vasomotion abnormalities regardless of the presence or absence of obstructive coronary artery disease. Coronary vasomotion is regulated by multiple mechanisms that include the endothelium, vascular smooth muscle cells (VSMCs), myocardial metabolic demand, autonomic nervous system and inflammation.3–5 Coronary vasomotion abnormalities, such as coronary artery spasm, play important roles in the pathogenesis of diverse cardiovascular diseases.
Maseri et al. have pointed out that coronary vasomotion abnormalities modulate residual coronary flow in patients with chronic stable, unstable and variant angina, and syndrome X.6,7 Over the years, several animal models of the spasm have been developed to explore its central mechanism.8–10
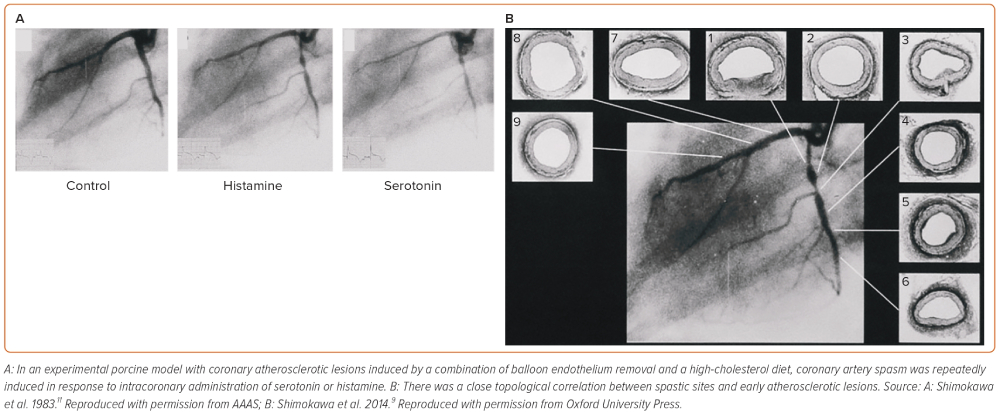
It is deemed that atherosclerotic lesions are angiographically predisposed to coronary artery spasm. Based on this clinical observation, we first used an experimental porcine model with coronary atherosclerotic lesions induced by a combination of balloon endothelium removal and a high-cholesterol diet.10–12 In this model, coronary artery spasm was repeatedly induced in response to intracoronary administration of serotonin or histamine in mild atherosclerotic lesions (Figure 1), providing the first experimental evidence for the close link between coronary artery spasm and coronary atherosclerosis.11,12 Ten years later, this experimental finding was confirmed in patients who had undergone coronary balloon angioplasty and later in those with vasospastic angina (VSA).13,14 Importantly, Maseri and his group reported the enhanced local constrictor response to a variety of stimuli acting on different receptors of ergonovine and acetylcholine in patients with variant angina.15,16 These alterations of local hyperreactivity are in the same coronary segment over weeks and months, although remaining non-spastic coronary segments are equally exposed to the drug.17,18 Lines of evidence from our basic studies have confirmed that the local hyperreactivity of VSMC rather than an abnormal specific agonist–receptor interaction plays a key role in inducing coronary artery spasm regardless of stimuli in pigs in vivo.9
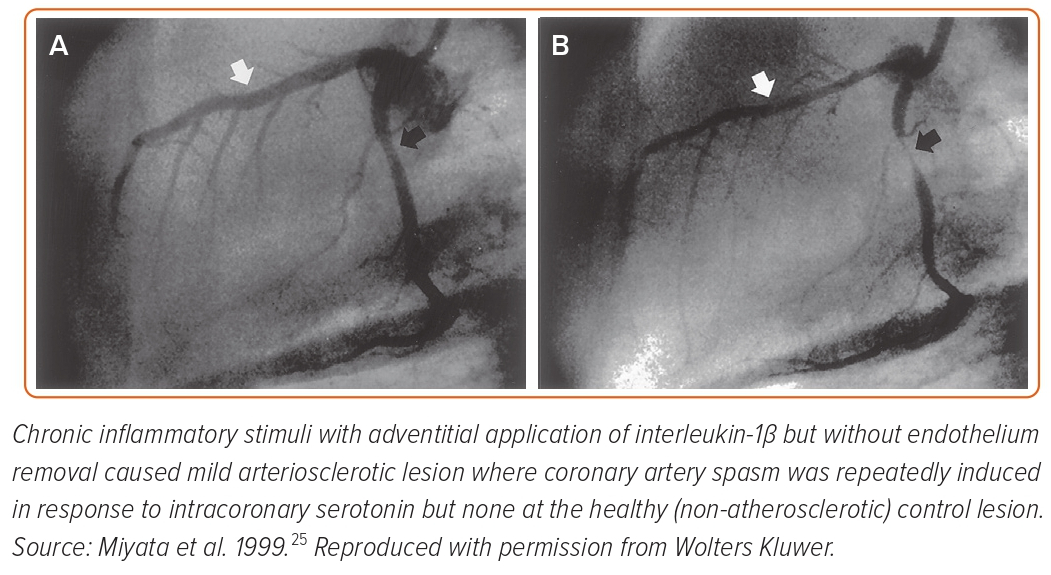
Seminal pathological studies have shown that spastic coronary segments have abundant adventitial inflammatory cells and perivascular nerve lesions.19,20 To determine the roles of endothelial dysfunction and VSMC hypercontraction separately, we developed a second porcine model with chronic adventitial inflammation but without endothelium removal.21–25 In this model, chronic inflammatory stimuli with adventitial application of interleukin-1β (IL-1β) caused mild arteriosclerotic coronary lesions, where coronary artery spasm was repeatedly induced in response to intracoronary serotonin or histamine but not at the control (non-atherosclerotic) lesion (Figure 2).21 These results were consistent when animals were chronically treated with IL-1α and tumour necrosis factor-α for 4 weeks.24 Importantly, in this porcine model, endothelial vasodilator functions were fully preserved as expected.25 Thus, we concluded that VSMC hypercontraction associated with adventitial inflammatory changes plays a major role in the pathogenesis of the spasm.9,21
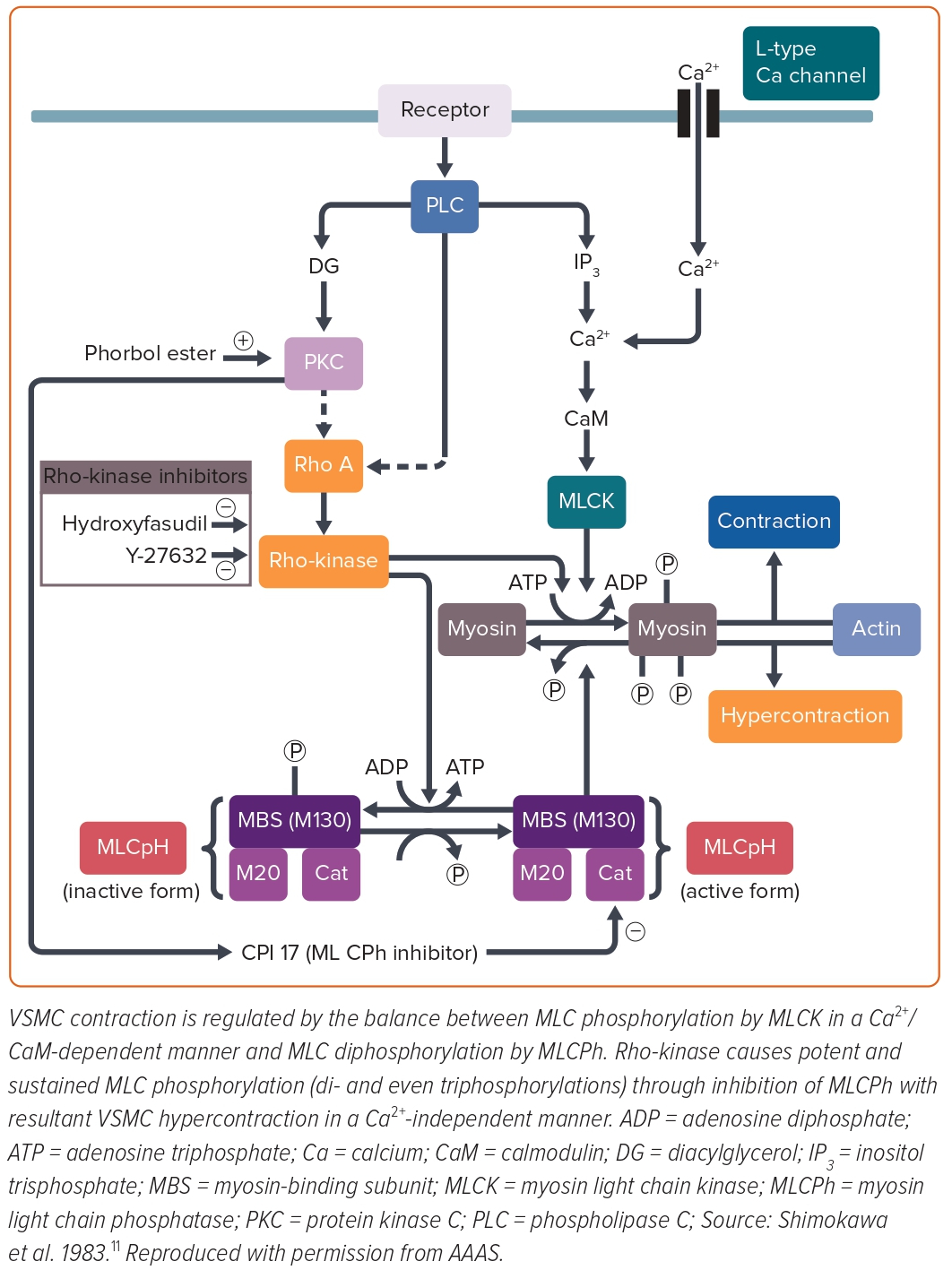
Mechanistically, contractile responses of VSMC were augmented at the spastic coronary segment in the porcine models of coronary artery spasm, which was later confirmed in a patient with variant angina.26–28 However, in the first porcine model with balloon endothelium removal and a high-cholesterol diet, the spastic segment showed no alteration of the VSMC vasocontractile response to incremental concentrations of calcium ions (Ca2+), suggesting that the key mechanism for coronary spasm other than Ca2+ sensitivity of contractile proteins per se is present somewhere between receptors and contractile proteins in the signal transduction pathway for VSMC contraction (Figure 3).26 Furthermore, in the second porcine model with chronic IL-1β treatment for 4 weeks, coronary hypercontraction is mediated by the 5-hydroxytryptamine (5-HT)2A serotonergic receptor, whereas the expression or functions of the 5-HT2A receptor are not significantly altered when compared with normal coronary artery segments.29 These results suggested that the key mechanism for coronary artery spasm is present somewhere below the receptors and above the contractile proteins in the signal transduction pathway for VSMC contraction. Furthermore, direct activation of protein kinase C (PKC) causes coronary artery spasm and inhibition of PKC suppressed the spasm in both porcine models.30,31 The inhibitory effects of PKC inhibitors were not evident in the coronary contractions in response to prostaglandin F2α.30,31 These results indicated that the PKC-mediated pathway is substantially involved in the pathogenesis of coronary spasm (Figure 3). VSMC contraction is triggered by Ca2+/calmodulin-activated myosin light chain kinase (MLCK) with subsequent phosphorylation of the 20-kDa regulatory MLC (Figure 3).32 Intriguingly, the porcine model with chronic IL-1β treatment revealed that MLC monophosphorylation was enhanced at the spastic coronary segment and that there was a positive correlation between the serotonin-induced coronary vasoconstrictions and MLC monophosphorylation and also diphosphorylation.27 MLC diphosphorylation occurs only in the actively growing cells and the spastic coronary segment.24,31 The increase in intracellular Ca2+ levels causes MLC monophosphorylation alone, whereas inactivation of MLC phosphatase causes both mono- and diphosphorylation of MLC.33,34 Thus, MLC phosphatase activity appeared to be essential for the induction of MLC diphosphorylation in VSMCs, which is known to induce potent and sustained VSMC contraction compared with MLC monophosphorylation.32
Rho, a small guanosine triphosphate, is involved in the GTP-enhanced Ca2+ sensitivity of VSMC contraction.35 Rho regulates MLC phosphorylation through its target Rho-kinase and the myosin-binding sub-unit (MBS) of myosin phosphatase.36 Activated Rho causes Rho-kinase activation and then activated Rho-kinase phosphorylates the MBS with resultant inactivation of myosin phosphatase. An active form of Rho-kinase enhances MLC phosphorylation and induces VSMC contraction (Figure 3).35,37,38 We identified that fasudil, which is clinically used for the treatment of cerebral vasospasm after subarachnoid haemorrhage in Japan, is metabolised to hydroxyfasudil which specifically inhibits both isoforms of Rho-kinase – ROCK1 and ROCK2 – but not other kinases such as MLCK or PKC.39–42 Indeed, hydroxyfasudil shows dose-dependent inhibition of serotonin-induced coronary spasm in the porcine model with chronic IL-1β treatment through suppression of MLC mono- and diphosphorylations.43 These results indicate that the Rho/Rho-kinase pathway plays an important role in the enhanced MLC phosphorylations in the spastic coronary artery, suggesting that inhibition of the Rho-kinase pathway may be a novel therapeutic target of coronary artery spasm (Figure 3).
In our porcine model, real-time (RT)-polymerase chain reaction (PCR) analysis demonstrated that expressions of Rho-kinase and RhoA mRNA, as well as the extent of MBS phosphorylation, were significantly upregulated in the spastic segment compared with the control.44 Similar to hydroxyfasudil, another Rho-kinase inhibitor of Y-27632 inhibited not only serotonin-induced hypercontractions in vivo and in vitro but also the increase in MBS phosphorylations.45 Thus, we concluded that upregulated and activated Rho-kinase plays a key role in inducing VSMC hypercontraction by inhibiting MLC phosphatase through MBS phosphorylation in porcine models in vivo (Figure 3).8–10,43,44
Epicardial coronary spasm is the local phenomenon that is unique to a given site of the coronary artery, whereas endothelial dysfunction is rather systemic. Furthermore, vasodilating responses to endothelium-dependent vasodilators, such as bradykinin or substance P, are fairly preserved at the spastic coronary segment in patients with variant angina.25 Indeed, endothelial dysfunction does not explain the high prevalence of coronary spasm from night to early morning, whereas the circadian variation of Rho-kinase activity (a molecular switch of VSMC contraction) has been well studied.46 Furthermore, nitrates exert acute inhibitory effects on coronary spasm by VSMC relaxation but have no acute effect on endothelial dysfunction.47 For these reasons, we consider that VSMC hypercontraction, rather than endothelial dysfunction, plays a primary role in the mechanism of coronary artery spasm.
We further reported that remnant lipoproteins from patients with sudden cardiac death enhance coronary vasospastic responses through upregulation of Rho-kinase in pigs in vivo and that sustained elevation of serum cortisol levels sensitises coronary vasoconstricting responses to restraint stress in pigs in vivo.48,49 Inflammatory stimuli, such as angiotensin II and IL-1β, upregulate Rho-kinase expression and activity in human coronary vascular smooth muscle cells in vitro, for which oestrogen mediated by an oestrogen receptor and nicotine exert divergent inhibitory and stimulatory effects on Rho-kinase expression, respectively.50,51 The significant interaction between ageing – possibly due to the mechanisms of the menopause – and smoking for women with VSA was followed in a study by the Japanese Coronary Spasm Association (JCSA) using large-scale cohort data.47,50,52–54 Clinical studies revealed that intracoronary fasudil exerts an inhibitory effect not only on epicardial coronary spasm but also microvascular spasm in patients in vivo.55,56 Intracoronary fasudil is also effective in relieving intractable coronary spasm resistant to nitrates and Ca-channel blockers, and this effect is consistently observed for acetylcholine-induced coronary hyperconstricting responses immediately after PCI.57–58 More recently, intracoronary fasudil significantly improved PCI-related MI expressed by corrected thrombolysis in MI (TIMI) frame count and TIMI flow grade.59 The role of the Rho-kinase pathway is extensive in a wide variety of cardiovascular diseases, such as the development of coronary atherosclerosis and in-stent restenosis lesions after PCI, cardiac allograft vasculopathy, heart failure, essential hypertension, pulmonary arterial hypertension and aortic aneurysm.60–70
In their review, Maseri et al. mentioned that there is ethnic heterogeneity in the coronary artery vasomotor reactivity.71 In this paper, Japanese patients with variant angina appeared to have diffuse and multivessel spasm as compared with white patients. Furthermore, Japanese patients with a recent MI had a higher incidence of spasm induced by provocative testing with acetylcholine, ergonovine, methylergonovine or serotonin, than white patients. This observation was later confirmed by the same group by conducting the same spasm provocative tests with acetylcholine in Japanese and Italian patients with recent MI.72 However, contrary to these findings, several European groups have recently reported a relatively higher incidence of inducible coronary artery spasm in larger cohorts of white patients who underwent coronary angiography and were found to have unobstructed coronary arteries.73,74 Likewise, the most recent study from an international and prospective cohort for microvascular angina by the COVADIS group has underlined that there was no difference in prognosis for different ethnic groups.75 Therefore, ethnic differences in coronary vasomotion abnormalities may be less relevant than we considered previously.
Rho-kinase Activity in Circulating Leukocytes as a Marker of Vasospastic Angina
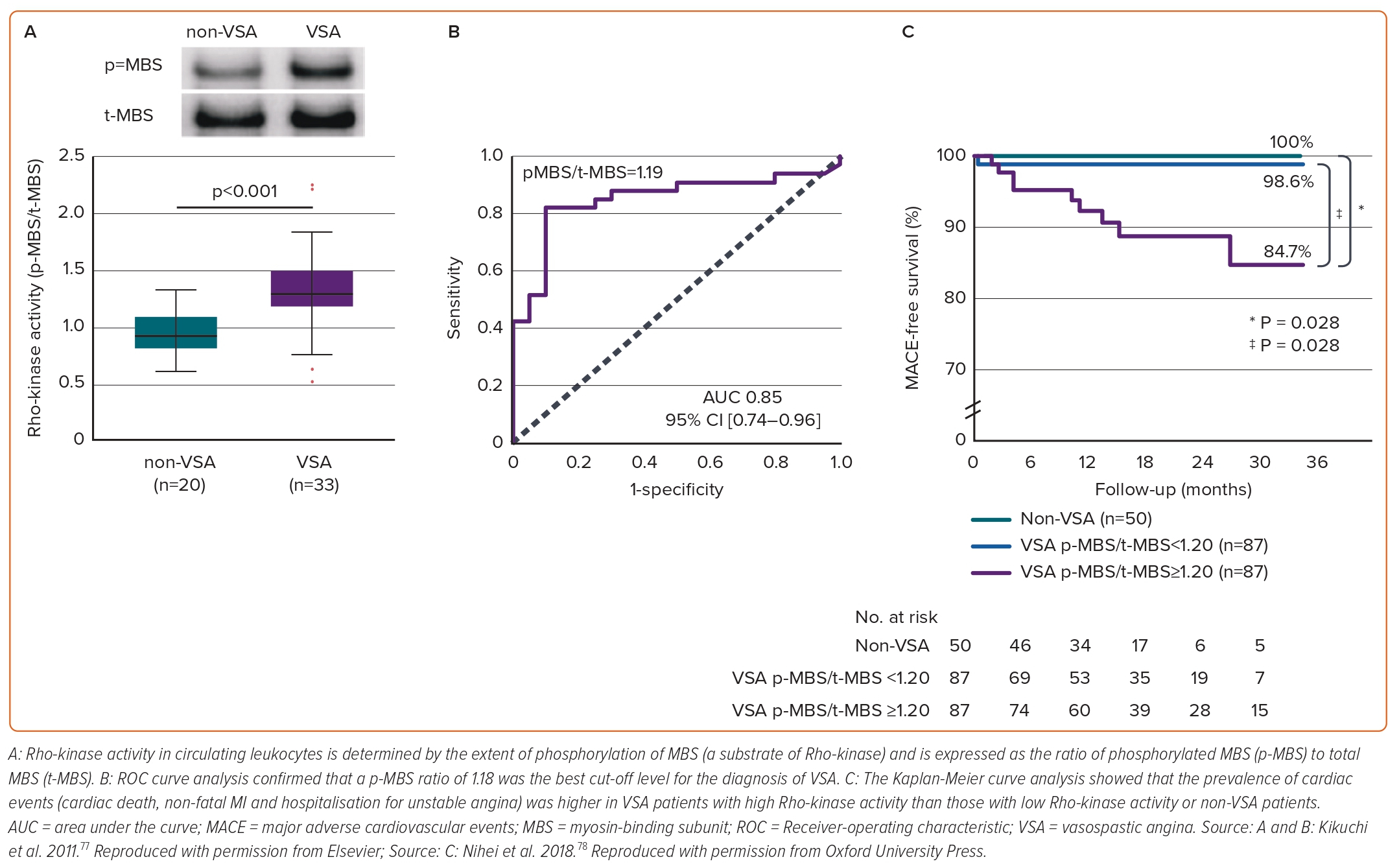
In addition to the relevant clinical implication of selective Rho-kinase inhibitors,39–42 Rho-kinase activity in circulating neutrophils holds promise as its potential to predict disease activity and long-term prognosis of VSA. Rho-kinase activity is determined by the extent of phosphorylation of MBS, a substrate of Rho-kinase and then Rho-kinase activity in circulating neutrophils is expressed as the ratio of phosphorylated MBS (p-MBS) to total MBS (t-MBS) (Figure 4A).76 A p-MBS ratio of 1.18 was identified as the best cut-off level to predict the diagnosis of VSA (Figure 4B).76 Findings were followed by lines of evidence that Rho-kinase activity in circulating neutrophils in VSA patients was temporally enhanced associated with disaster-related mental stress during the 2011 earthquake and tsunami in Asia, and that the Rho-kinase activity corresponds to distinct circadian variation of VSA, peaking in the early morning and is associated with altered coronary vasomotor responses and autonomic activity.46,77 Maseri and his group suggested that parasympathetic withdrawal may be a part of the mechanism of spontaneous coronary artery spasm.5 Rho-kinase activity of VSA patients in the early morning was significantly correlated with parasympathetic nervous activity from midnight to early morning.46 Furthermore, patients with VSA with higher Rho-kinase activity (≥1.20) had a poor long-term prognosis (Figure 4C). In the study, a p-MBS ratio of 1.24 was identified as the best cut-off level to predict future cardiac events in VSA patients.78 Of note, a combination of Rho-kinase activity with the JCSA risk score saw a pronounced improvement of the prognostic value with either alone.47,78
Coronary microvascular dysfunction (CMD) has been emerging as an exacerbating factor of long-term prognosis of cardiovascular patients.79,80 The first international study from the COVADIS group has demonstrated that major cardiovascular adverse events (MACE), including cardiovascular death, non-fatal MI, non-fatal stroke and hospitalisation due to heart failure or unstable angina, occurred irrespective of sex or ethnicity.75,81 In the context of a novel biomarker for VSA and comorbid CMD, we have demonstrated that CMD determined by the increased index of microcirculatory resistance (IMR) >18 heightened the MACE rate of VSA patients.82 In such patients, intracoronary administration of fasudil significantly ameliorated IMR, which suggests a possible role of the Rho-kinase pathway in CMD.82 Another landmark study has reported that the measured plasma concentration of serotonin from the coronary artery and coronary sinus was highest in VSA patients with CMD, while there was no difference in the VSA without CMD versus non-VSA groups.83 Fractional flow reserve (FFR), as known as a marker for evaluating the degree of flow-limiting coronary stenotic region, successfully extracted the high-risk population among VSA patients, particularly with significant organic stenosis.84 Current guidelines and expert consensus recommends spasm provocation tests with additional interventional approaches even when no organic stenosis is found angiographically.85,86 The approaches that we will summarise are a promising avenue to fully elucidate the pathogenesis of coronary artery spasm in patients with VSA.
Roles of Adventitial Inflammatory Changes in Enhanced Coronary Vasoconstricting Responses
Ample evidence has been accumulated to elucidate the detailed mechanisms of coronary artery spasm in vivo. Using the porcine models with inducible coronary artery spasm, we found the role of coronary adventitial inflammatory changes – known as the ‘outside-in’ pathway – in the pathogenesis of coronary artery spasm and extensively in coronary atherosclerosis.21,44,60 In brief, coronary adventitia harbours a wide range of cellular and subcellular components, such as nutrient blood vessels (termed vasa vasorum), perivascular adipose tissue (PVAT), autonomic nervous system, and lymphatic vessels that drain adventitial inflammatory cells.87–94 We have demonstrated that these adventitial residents have interactions with each other, all of which are involved in coronary hyperconstricting responses after drug-eluting stent (DES) implantation in pigs in vivo (Figure 5).87–93
The first observation was that inflammatory changes were potent in the histology of coronary adventitia isolated from the porcine coronary segment with first-generation DES implantation compared with bare-metal stent implantation.95,96 Adventitial inflammation was significantly reduced when implanting newer-generation biocompatible DES in pigs.87,97 Experimental micro-CT revealed that adventitial vasa vasorum formation was augmented around the coronary segment implanted with first-generation DES (Figure 5) as compared with the segment with biocompatible DES.87 The extent of vasa vasorum formation was proportional to that of M1-type macrophage and mast cell infiltrations, indicating the important role of vasa vasorum as an inflammatory conduit.87 Furthermore, these adventitial inflammatory changes were associated with DES-induced coronary hyperconstricting responses in vivo.87,97 PVAT surrounds the coronary arterial wall and is thought to be an origin of various inflammatory cytokines, such as adipokines.91 We then demonstrated that 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) is useful for evaluating the magnitude of coronary PVAT inflammation in pigs after DES implantation ex vivo and in vivo (Figure 5).90 In the novel approach with 18FDG-PET, the extent of PVAT inflammation is expressed as a target-to-background ratio, the standardised uptake value (SUV) corrected for blood activity by dividing the average blood SUV estimated from the ascending aorta.90 Importantly, 18FDG-PET-derived PVAT inflammation was strongly associated with DES-induced coronary hyperconstricting responses in vivo.90 We further confirmed that the autonomic nervous system existing in the coronary adventitia was associated with exacerbated inflammation and enhanced vasoconstriction via the kidney-brain-heart axis in the same porcine model.92 More recent studies underline that impairment of cardiac lymphatic drainage function after DES implantation contributes to sustained adventitial inflammation, medial VSMC hypercontraction and resultant coronary hyperconstriction through activation of Rho-kinase in pigs in vivo.93,94
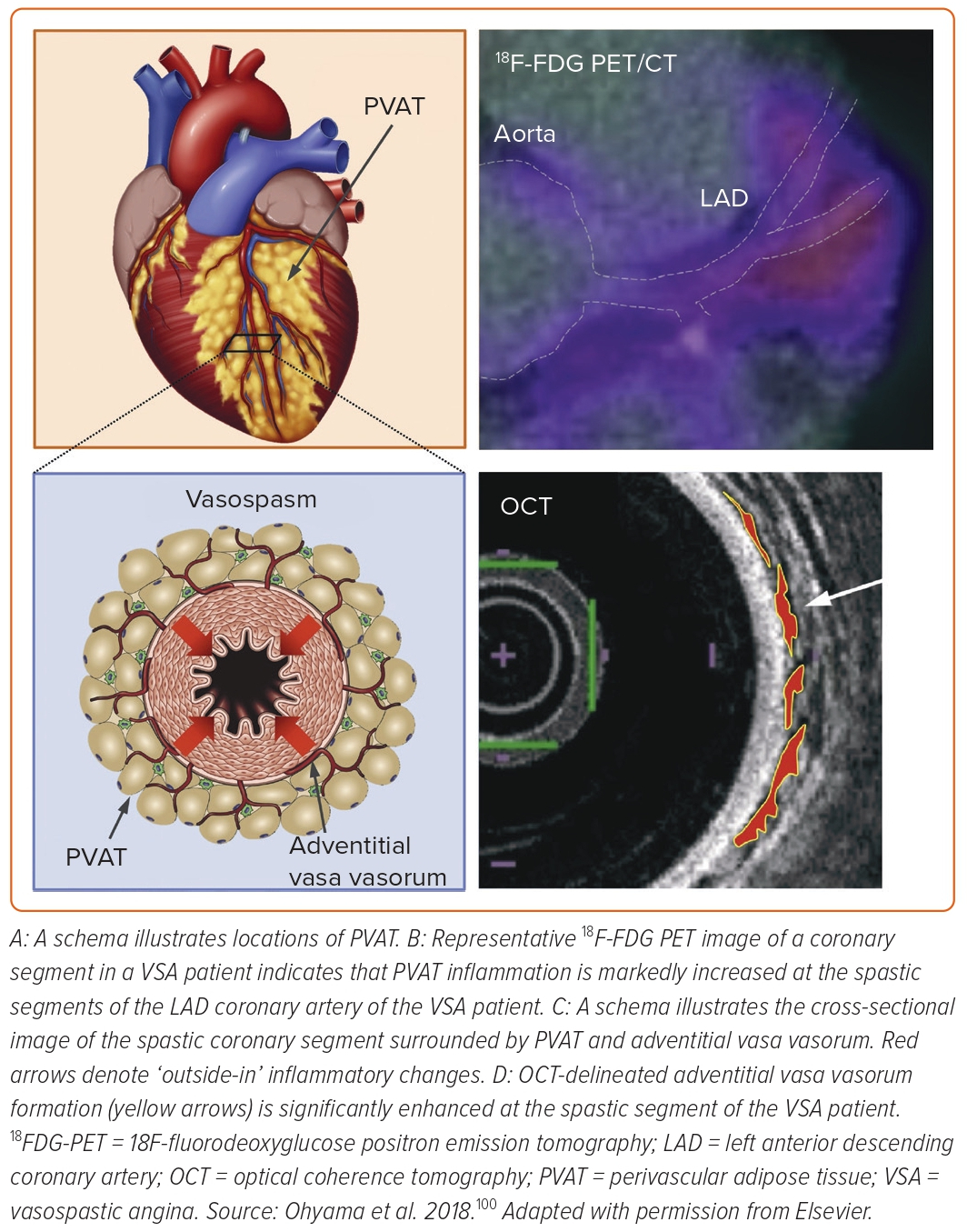
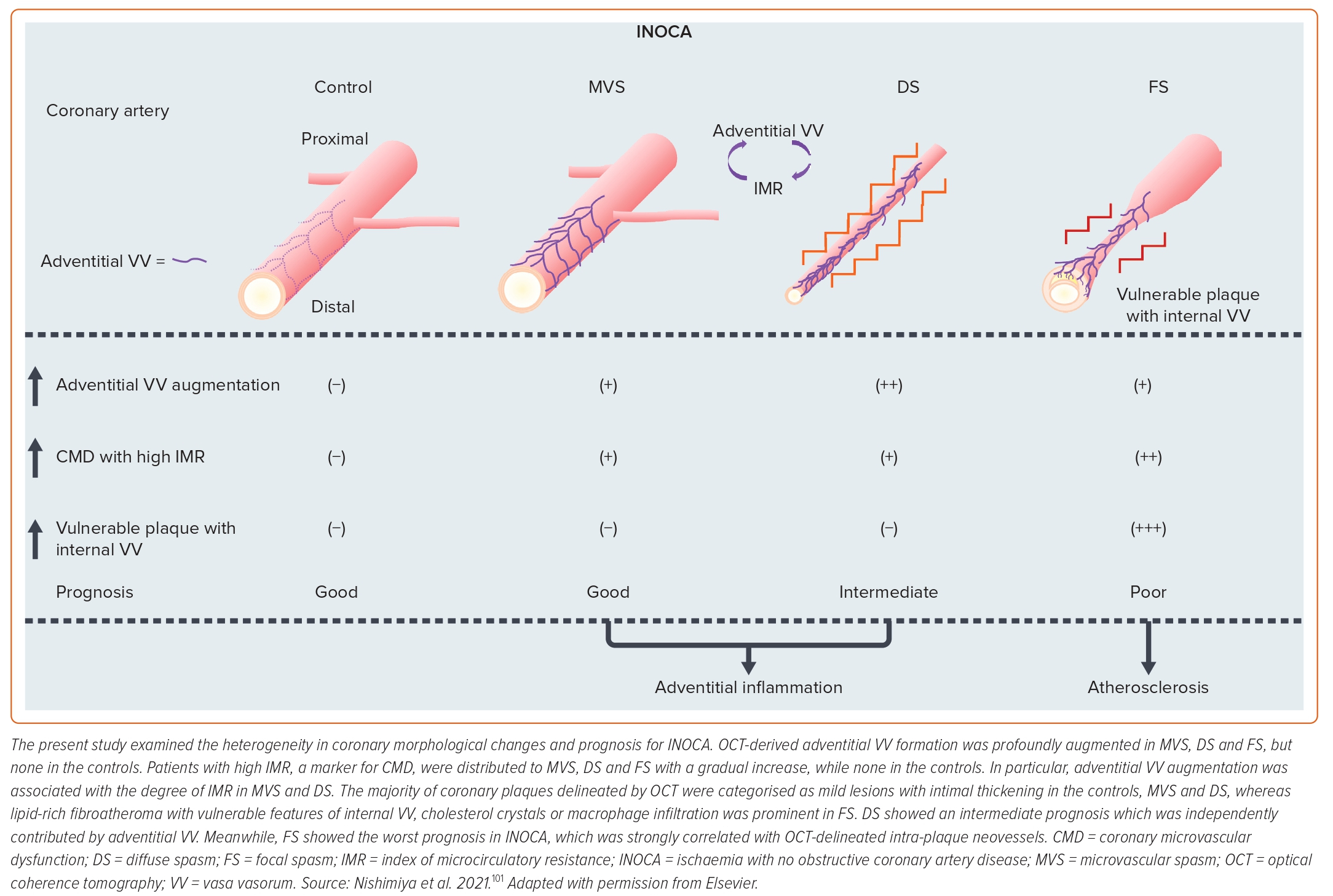
We further examined the role of coronary adventitial inflammation in the clinical settings by using a multimodality imaging approach with optical coherence tomography (OCT) and 18FDG-PET.98–101 OCT is capable of visualising coronary adventitial vasa vasorum in pigs and humans and OCT-delineated adventitial vasa vasorum (VV) formation was significantly enhanced at the spastic segments of VSA patients as compared with those of the control subjects (Figure 5).98–101 Maseri et al. suggested that focal coronary artery spasm is a distinct condition characterised by focal coronary constriction sufficient to cause transient total or sub-total coronary occlusion.6 Indeed, when grouping 335 patients with ischaemia and no obstructive coronary artery (INOCA) into the group of non-cardiac controls, microvascular spasm, diffuse epicardial spasm and focal epicardial spasm, the group of focal epicardial spasm had the worst prognosis followed by the group with diffuse epicardial spasm (Figure 6).101 The extent of adventitial vasa vasorum formation visualised by OCT was increased in patients with microvascular spasm as compared with controls, and peaked in those with diffuse spasm. The augmentation of adventitial vasa vasorum was positively correlated with the level of IMR, indicating the possible link between coronary adventitial inflammation and microcirculatory resistance.101 In particular, the vasa vasorum that links to intramural layers, termed as intra-plaque neovessels, showed a relevant prognostic value among patients with focal coronary artery spasm.101 Also, 18FDG-PET-derived coronary PVAT inflammatory changes were more extensive at the spastic coronary segments of VSA patients compared with those of control subjects (Figure 5).100 The lines of basic and clinical studies strongly support the notion that coronary adventitial inflammation could be a novel therapeutic target for coronary artery spasm.
When treating coronary adventitial inflammation, it is ideal to directly administer anti-angiogenic and/or anti-inflammatory agents to patients. However, systemic use of these drugs may often cause serious side effects.102,103 The anti-angiogenic strategy for vasa vasorum may cause intramural haemorrhage as a consequence of increased vascular permeability.104 We then introduced that catheter-based renal sympathetic denervation can effectively suppress DES-induced coronary hyperconstricting responses via the kidney-brain-heart axis in pigs in vivo.92 However, this approach requires multiple invasive steps. Thus, we focused on the low-intensity pulsed ultrasound (LIPUS) therapy as a novel non-invasive therapeutic option for coronary adventitial inflammation and coronary artery spasm.105–112 Since the intensity of ultrasound used in the LIPUS therapy is below the upper limit of acoustic output standards for diagnostic devices, it causes no compression, heat, or discomfort.105–112 Our recent study has demonstrated that the LIPUS therapy suppresses DES-induced coronary hyperconstricting responses in pigs in vivo by suppressing Rho-kinase activity.94 The detailed molecular mechanisms of the LIPUS are that it enhances cardiac lymphatic vessel formation associated with endothelial nitric oxide synthase upregulation with no effects on the vasa vasorum, and that the enhancement of lymphatic vessel formation improves inflammatory drainage function, helping abrogate adventitial inflammation and Rho-kinase activity.94 In addition to coronary artery spasm, PVAT inflammation has been demonstrated in human coronary atherosclerotic lesions in vivo.113 Taken together, the LIPUS therapy could be a novel and safe therapeutic approach for a broad spectrum of cardiovascular disease in clinical practice.114
Conclusion
Recent clinical trials have highlighted that PCI in patients with stable angina provides limited additional prognostic benefits on top of optimal medical therapy.1,2 In the era after ISCHEMIA and REVIVED-BCIS2, coronary vasomotion abnormalities, regardless of obstructive or non-obstructive arterial segments, will increase in clinical significance year by year. Unveiling the mechanism of coronary artery spasm, particularly in the clinical setting, will be a next step to mitigate this important disorder.