The most typical and well-defined clinical condition caused by coronary artery spasm (CAS) is Prinzmetal or variant angina.1 Variant angina is characterised by recurrent angina attacks that usually last 5–10 min, but sometimes only 30–60 s, and occur at rest, frequently at night, and usually in the absence of apparent triggering factors. However, exercise may trigger CAS in approximately 25% of patients.2 The ECG recorded during angina attacks typically shows ST-segment elevation, indicating transmural myocardial ischaemia, although ST-segment depression may occur in some patients/episodes, possibly because of subocclusive spasm and/or the presence of collateral flow. In these patients, CAS may occur at the level of one or more coronary segments (focal spasm) or diffusely involve one or more epicardial arteries. Furthermore, CAS may occur at the level of significant or non-significant coronary artery plaques, as well as in angiographically normal coronary segments.3–6 In most cases, a diagnosis of variant angina can be made on the basis of clinical and ECG (standard, ambulatory monitoring, exercise) findings, only occasionally requiring provocation tests, the most frequently used of which are now intracoronary acetylcholine (ACh) or intracoronary/intravenous ergonovine tests.2
Although variant angina is the most typical clinical presentation of CAS, CAS can also be responsible for other different clinical presentations, including an acute coronary syndrome (ACS; e.g. the sudden appearance of one or a few episodes of chest pain at rest with ischaemic ECG changes, with or without an increase in biomarkers of myocardial necrosis) or predominantly exercise-induced angina.7–10 In these patients, a diagnosis of CAS is difficult to make based on clinical and ECG findings only, particularly when obstructive coronary artery disease (CAD) is demonstrated on coronary angiography and only ST-segment depression and/or T wave changes are detected on the ECG. In these patients, CAS as a cause of the clinical syndrome is usually only considered when normal or near-normal coronary arteries are found on angiography and is identified as the mechanism causing the disease based on positive provocation tests (ACh or ergonovine).11
This broad spectrum of CAS-related clinical presentations is usually gathered under a unique clinical syndrome, defined as vasospastic angina (VSA).11 Most studies assessing clinical characteristics and outcomes of CAS have variably included patients with these different forms of VSA. Accordingly, the term ‘VSA’ is used in this article to indicate any type of CAS-related syndrome, whereas the term ‘variant angina’ is used when discussing findings obtained in patients with characteristics of this typical CAS-related syndrome.1–6
It should be noted that it is not clear whether the different forms of clinical syndromes included in the VSA diagnosis have the same prognostic implications and the same symptomatic benefits from established CAS therapy. Although a positive provocation (usually ACh) test for epicardial spasm identifies patients with increased vessel reactivity, it is not clear whether it invariably identifies the actual mechanism underlying the clinical syndrome in the patient, which may, in fact, be related to some other mechanism (e.g. an additional coronary microvascular spasm) that could cause symptoms similar to those induced by CAS.12,13
General Considerations for CAS Management
Some relevant points should be kept in mind when considering the management of CAS.
First, transmural myocardial ischaemia induced by occlusive CAS may result in severe complications, such as ventricular tachyarrhythmias (or even bradyarrhythmias), which may result in syncope and sudden death,14–16 or acute MI (AMI),2,3 which may result from prolonged occlusive spasm. Thus, effective CAS prevention does not merely improve angina symptoms, but may also be life saving for some patients.
Second, because most episodes of CAS-induced myocardial ischaemia are silent, treatment should ideally aim to abolish not only angina episodes, but also episodes of silent myocardial ischaemia detected by 24/48-h ECG Holter monitoring.17 This is particularly important in patients with features of high risk, including those with CAS-related arrhythmias, prolonged CAS or CAS slowly responding to short-acting nitrate administration, in whom the protection afforded by CAS therapy should also be tested by provocation tests.
Third, treatment should be started as soon as the diagnosis of CAS is made because most acute events (up to 75%), including sudden death, occur in the first 1–3 months from symptom onset; thus, a delay in starting an efficacious therapy may be associated with the occurrence of clinically relevant events.2,18
Fourth, although medical treatment is usually highly effective in preventing CAS and its serious complications (see below), the inclusion in study populations of patients presenting findings of high risk of cardiovascular events independent from CAS and not influenced by anti-CAS therapy (e.g. the presence of multivessel coronary artery disease [CAD]) might negatively bias the efficacy of CAS therapy on global clinical outcomes.
Finally, effective vasodilator therapy for CAS-related angina attacks should be continued indefinitely, particularly in patients with high-risk factors; indeed, therapy withdrawal may have dramatic consequences due to a significant rate of CAS recurrence.19
Treatment
Angina attacks caused by CAS usually cease spontaneously or respond quickly to sublingual/buccal short-acting nitrates, such as nitroglycerin 0.3 mg or isosorbide dinitrate (ISDN; 5 mg). Only in rare cases is the administration of intravenous nitrates or calcium channel blockers (CCBs) necessary to resolve CAS.
Calcium Channel Blockers
The non-specific arterial dilator CCBs form the cornerstone of treatment for the long-term prevention of CAS. The efficacy of these drugs in preventing CAS, and therefore abolishing, or consistently decreasing, angina attacks, has been clearly demonstrated in several studies and is a common experience in clinical practice (Figure 1).20–25
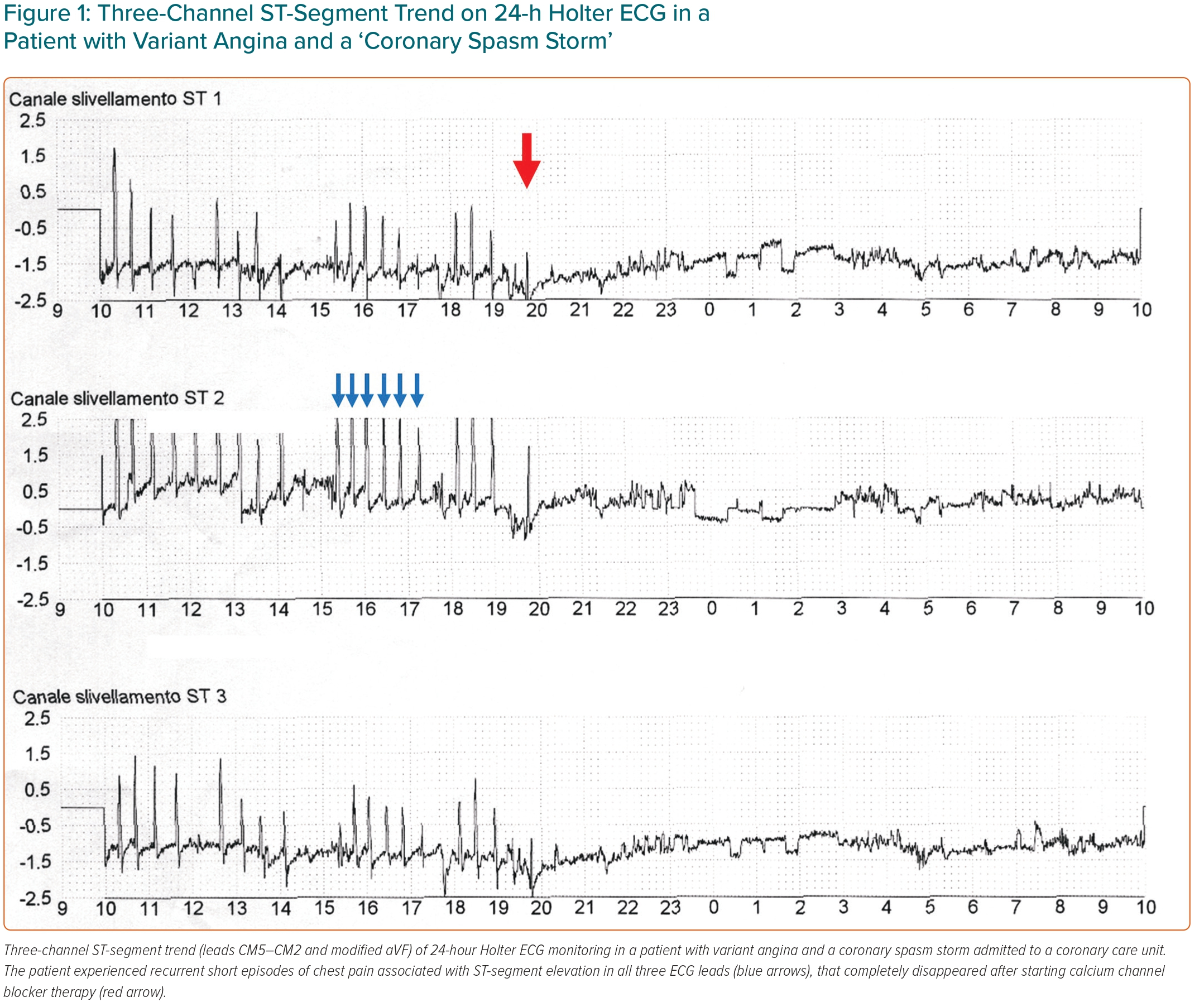
Both dihydropyridine (DHP) and non-DHP drugs can be used. Typical average doses of CCBs include verapamil or diltiazem 120 mg in slow-release (sr) formulations given two to three times a day, nifedipine 20–30 mg or twice a day or 10 mg amlodipine once daily. These standard doses of CCBs fully prevent angina attacks in 80–90% of patients.20–25
CCBs at standard doses are usually well tolerated. The most frequent unwanted effect is ankle oedema, with constipation being another bothersome side effect. DHP CCBs may also cause reflex tachycardia and hypotension, which are attenuated when long-acting CCBs (e.g. amlodipine) or sr formulations of short-acting drugs (e.g. nifedipine) are used. Conversely, bradycardia may preclude the use of full doses of non-DHP drugs.
Importantly, observational follow-up studies have shown that control of CAS by CCBs translates into significant improvements in clinical outcomes in patients with variant angina. In a first study, Waters et al. followed 169 patients with variant angina (37% of whom had coronary stenoses >70%) for a mean period of 15.3 months.26 Although CCBs did not significantly reduce mortality in the entire population, the use of nifedipine, diltiazem or verapamil improved survival from AMI compared with perhexiline or long-acting nitrate treatment among patients with no multivessel disease (92% versus 67%; p<0.005).26 In a similar study of 245 patients with variant angina (40% of whom had coronary stenoses >70%) followed for a mean of 80.5 months, there was a significant association between the use of CCBs (diltiazem and/or nifedipine) and significant improvement in survival and a lower occurrence of AMI compared with no CCB therapy.27 Thus, these two pivotal studies demonstrated clear benefits of CCBs on clinical outcomes in patients with variant angina.
Some studies have suggested that there may be differences in the efficacy of different CCBs on CAS. A meta-analysis of four studies, including 1,997 patients with VSA, suggested that benidipine (currently only available in Asia) was more effective than nifedipine, amlodipine or diltiazem in improving symptoms and/or clinical outcomes (cardiac death, AMI, heart failure, stroke and aneurysm).28
However, in a recent multicentre study including 2,960 patients with VSA, there was no difference at the 3-year follow-up in clinical events (death, ACS, symptomatic arrhythmias) between patients treated with first-generation CCBs (diltiazem, nifedipine) and those treated with second-generation CCBs (benidipine, amlodipine), despite benidipine showed better control of symptoms than diltiazem.29
These studies have several limitations. First, they are retrospective, not randomised, studies; thus, it is not possible to exclude the possibility that the results are biased by confounding factors in terms of the choice of CCB and the dosage used. Importantly, the unexpected lack of effects of CCBs, other than benidipine, on symptoms in the study of Nishigaki et al. may suggest some bias in patient selection.28 Overall, CCBs can be considered similarly effective on angina symptoms and clinical outcomes in CAS patients when appropriate drug dosages are prescribed.
Nitrates
Although short-acting nitrates constitute the standard treatment for ongoing angina attacks caused by CAS, the efficacy of long-acting formulations of these drugs for the long-term prevention of CAS is questionable. In two small randomised studies, ISDN showed an efficacy similar to that of nifedipine on angina attacks over a short-term follow-up in patients with variant angina.20,21 However, the efficacy of nitrates over long-term follow-up has not been well established.23,25
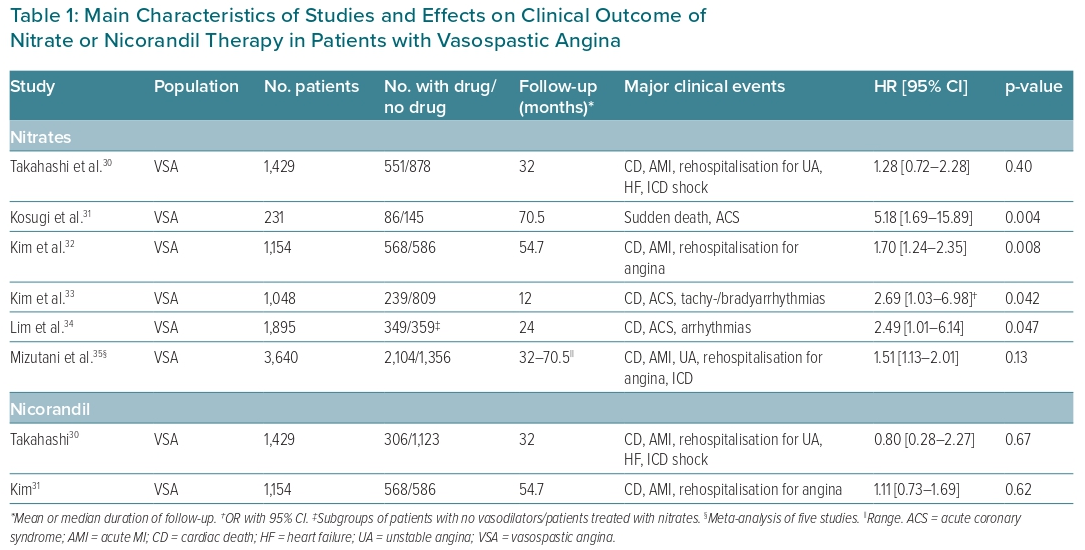
Importantly, some studies have suggested that the long-term use of nitrates does not improve clinical outcomes and may result in an increased occurrence of cardiovascular events in patients with VSA. As noted above, Waters et al. reported that the efficacy of treatment with nitrates was significantly lower than that of CCBs in reducing the occurrence of AMI in patients with variant angina.26 Several subsequent studies assessed the effects of nitrates on clinical outcomes in VSA patients, usually reporting increased or neutral risk of cardiovascular events compared with no nitrate therapy, as summarised in Table 1.30–35
The lack of beneficial effects of nitrates on CAS-related events may be related to a loss of their vasodilator effects on constricted epicardial arteries due to the development of tolerance.36 Furthermore, continuous exposure to nitrates may be associated with an impairment of endothelial function related to their release of nitric oxide molecules.37 These issues may be overcome, at least in part, by asymmetrical administration of short-lasting formulations of the drugs or the use of sr formulations, leaving a period of the day free from the effects of nitrates; this would restore vessel sensitivity to the vasodilator effects of subsequent nitrate administration and avoid the negative effects of continuous nitrate treatment.
However, it should also be noted that some of the negative results regarding the use of nitrates in observational studies of patients with VSA may be biased by the use of nitrates in patients with less effective symptom control, which may also suggest an increased risk of events.
Thus, in summary, although long-acting nitrates cannot be recommended as a first-line treatment for CAS, they can be recommended as second-line vasodilator drugs in addition to CCBs when the latter are not fully effective at their highest tolerated dose or their dosage cannot be increased due to the occurrence of side effects. In these cases, nitrates should be scheduled to cover the period of the day in which ischaemic episodes are expected to occur most frequently, due to the frequent circadian distribution of ischaemic attacks caused by CAS,38 leaving, as noted above, a period of 8–10 h of the day free from their effects.
Typical doses of nitrates are 10–20 mg ISDN or 10–20 mg isosorbide-5-mononitrate, both twice a day, or their sr formulations at doses of 40–50 mg given once daily.
Nitrates are usually well-tolerated drugs, with headache being their main side effect. Other rather common side effects include flushing, light-headedness, orthostatic hypotension and syncope. Nitrates should be avoided in patients with closed-angle glaucoma and those taking phosphodiesterase 5 inhibitors because of a high risk of severe hypotension.
Nicorandil
Nicorandil is a vasodilator drug with a double mechanism of action. Nicorandil primarily acts as a nitric oxide donor, thus having nitrate-like effects, but also acts as an opener of ATP-dependent K+ channels. Nicorandil has been reported to successfully resolve CAS refractory to CCB therapy in case reports and was found to be effective in preventing CAS in some small clinical studies.39,40 However, again, the long-term effects of nicorandil are not known. Furthermore, as shown for chronic nitrate therapy, observational studies have yielded disappointing results about the efficacy of nicorandil in reducing clinical events in patients with VSA, also resulting in an increased rate of clinical events when combined with nitrates, as summarised in Table 1.30,32,34
Thus, where available, nicorandil can be added to one or two CCBs, instead of nitrates, in case of symptom persistence. The usual dose is 10 mg nicorandil twice daily.
As for nitrates, headache is the most frequent side effect of nicorandil, with dizziness, flushing, gastrointestinal discomfort and hypotension also being common. In rare cases, nicorandil can cause gastrointestinal, skin, mucosal or eye ulceration, which requires immediate cessation of the drug.40,41 As for nitrates, nicorandil should be avoided in patients with low blood pressure and those taking phosphodiesterase 5 inhibitors.
Other Treatment Considerations
Although CAS is usually not related to identifiable triggers, in some patients it occurs in response to specific causes. These primarily include the use of substances that favour vasoconstriction, such as catecholaminergic and serotoninergic drugs, the chemotherapeutic 5-fluorouracil and illicit drugs, such as cocaine or cannabis.42–46 In these cases, the removal of the cause resolves the clinical picture. Importantly, when the administration of CAS-inducing drugs is important for the treatment of an underlying disease, controlling CAS with CCBs may allow the continued use of these drugs.47 Anaphylactic reactions are another specific mechanism responsible for the induction of CAS as a result of the release of histamine (Kounis syndrome);48 avoiding the allergenic substance will prevent CAS induction.
Importantly, the use of β-blockers was shown to exacerbate symptoms in some patients with variant angina.49 Indeed, β-blocker antagonism of the β2-adrenoceptor-mediated dilator effects of adrenergic substances may favour the induction of CAS by these substances acting on α-adrenoceptors. For this reason, β-blockers should possibly be avoided in CAS patients, although, when believed necessary for the patient, they can be administered with the appropriate use of CCBs.
Some studies have suggested that aspirin may exacerbate CAS, which could be related to inhibition of the endothelial production of prostacyclin.50 However, other studies and a recent meta-analysis have found a neutral effect of aspirin use on clinical outcomes in VSA patients.51,52 Accordingly, although there is no indication for the prescription of aspirin in VSA patients, it can be given safely when indicated for other clinical reasons.
In recent years some authors have suggested that CAS patients may benefit from statin therapy. Statins have been shown to improve clinical outcomes in patients with ischaemic heart disease due not only to their anti-cholesterolaemic action, but also other favourable effects, including anti-inflammatory and anti-oxidant actions and improvement of endothelial function, which may also favourably impact on CAS.53
In a prospective open-label study, Yasue et al. randomised 64 patients with normal coronary arteries and ACh-induced CAS to 30 mg/day fluvastatin plus CCBs or to CCBs alone.54 After 6 months of treatment, ACh-induced CAS during angiography was suppressed in 51.5% of patients in the statin group, compared with 21.2% of patients in the non-statin group (p=0.023). However, there was no significant difference in the suppression of angina symptoms (75.0% versus 70.4%; p=0.92) or ischaemic ECG changes (24.0% versus 22.7%) between the two groups.54
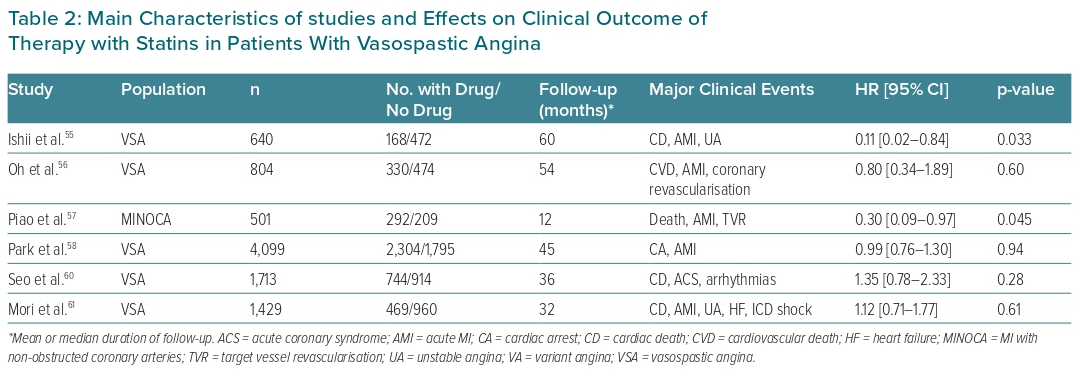
Subsequent studies focused on the effects of statins on clinical outcomes of VSA patients.55–61 The main results of these studies are summarised in Table 2. Although some studies reported positive results, on the whole the available data do not suggest that statins may improve clinical outcomes in this specific population of patients and therefore they cannot be recommended as a standard therapy in VSA patients.
General Measures
Although lifestyle modifications and behavioural and pharmacological control of modifiable risk factors are crucial in patients with obstructive CAD, these have limited implications for the control of CAS and the prevention of CAS-related cardiac events. Clinical studies have shown that only smoking and the use of alcohol show a significant association with the occurrence of CAS.62,63 Indeed, smoking and alcohol use have also occasionally been associated with triggering CAS.64,65 Thus, avoiding smoking and alcohol abuse should be recommended to all patients with CAS.
Conversely, despite their universal negative effects on vascular function, in particular endothelial function, there is no evidence of a significantly increased risk of CAS associated with other traditional cardiovascular risk factors, including hypertension, hypercholesterolemia and diabetes, although the control of these factors should also be recommended as a general intervention for cardiovascular risk prevention.
Refractory Coronary Artery Spasm
There is no well-established definition for refractory CAS. However, it may be diagnosed when CAS recurs despite a combination of standard doses of two CCBs (a DHP and a non-DHP drug) or a CCB and a long-acting nitrate (or nicorandil). The exact prevalence of refractory CAS is not well known, but stands between 10% and 15% among patients with variant angina and was reported to be 13.7% in a large Japanese study that included 921 patients with VSA.66
Although the refractoriness of CAS can be limited to brief periods, the treatment of patients with refractory CAS can be particularly demanding and even dramatic in the rare cases in which ischaemic attacks recur continuously, forming a real ‘CAS storm’, sometimes associated with life-threatening arrhythmias (possibly resulting in syncope or cardiac arrest) or severe impairment of left ventricle function (sometimes resulting in cardiogenic shock).
In a subset of these patients, adequate control of CAS is obtained with the use of increasing doses of CCBs, titrated up to the maximum tolerated dose (e.g. up to 960 mg/day diltiazem or verapamil) and possibly combined with nifedipine (up to 100 mg) or amlodipine (up to 20 mg). Similarly, high doses of nitrates can be added (e.g. 80 mg ISDN or isosorbide-5-mononitrate) or maximum tolerated doses of continuous intravenously administered nitrates (nitroglycerin, ISDN).
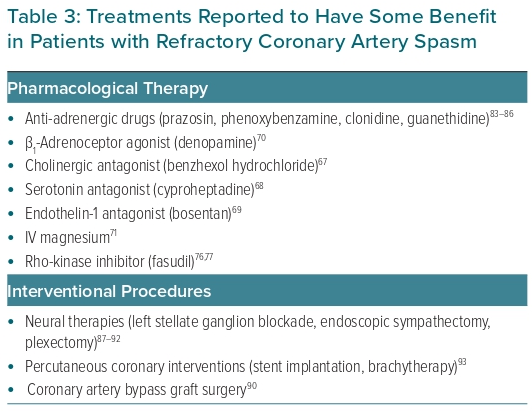
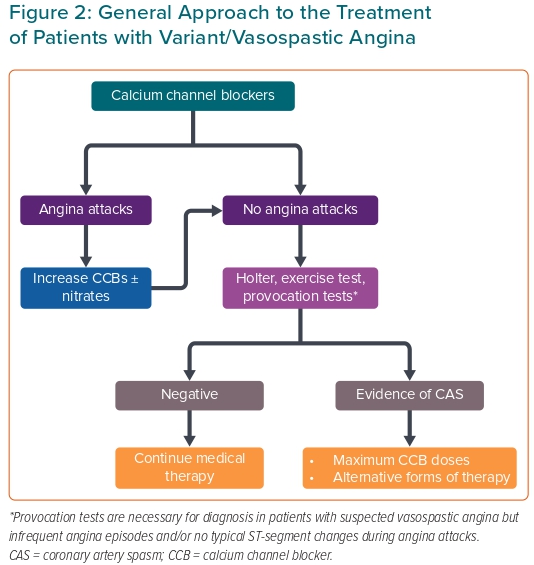
In the very few cases in which this treatment is insufficient or not tolerated, several additional forms of therapy have been proposed. However, because cases in which these severe forms of refractory CAS occur are rare, these therapies have only been tested in very few patients, or even reported as single case reports. Therefore, their use is empirical and no specific recommendations about their use can be given. However, any attempt may be considered acceptable when reported to be of potential benefit to the patient, in particular to control CAS storms. A list of the main treatments used in patients with refractory CAS is presented in Table 3. These treatments include antagonists of Rho-kinase, catecholamines and other vasoconstrictor agents, such as ACh, serotonin and endothelin-1, as well as drugs with vasodilator properties and non-pharmacological interventions.67–71 Some of these treatments are briefly discussed below. A flow chart for the management of patients with CAS is shown in Figure 2.
Rho-kinase Inhibitors
Rho-kinase plays a key role in the pathogenesis of CAS, both at the epicardial and microvascular level.72 Rho-kinase enhances myosin light chain phosphorylation through inhibition of myosin-binding subunit of myosin phosphatase, leading to vascular smooth muscle hyperreactivity to vasoconstrictor agents.73 Rho-kinase is upregulated in response to non-specific stimuli.74 Notably, the most potent stimulator of this enzyme is smoking, whereas an effective suppressor is oestrogen, which is in agreement with well-known epidemiological findings of VSA patients.75 Fasudil, a specific Rho-kinase inhibitor, is highly effective in preventing ACh-induced CAS and resultant myocardial ischemia.76 Furthermore, intracoronary fasudil has been shown to be effective not only in patients with epicardial coronary spasm, but also in approximately two-thirds of patients with microvascular angina.76,77 Unfortunately, the intravenous form of fasudil is available only in Japan, and no oral form of this Rho-kinase inhibitor is at present available for clinical use, even in Japan.
However, previous studies demonstrated that some drugs currently used in clinical practice exert indirect inhibitory effects on Rho-kinase through their anti-inflammatory actions, including eicosapentaenoic acid and ezetimibe, although the effects of these medications on CAS-related angina symptoms remain to be established. 74,78–80
Anti-Adrenergic Therapy
A high adrenergic state, favouring and/or triggering coronary spasm, has been suggested to play a significant pathophysiological role in most patients with VA, particularly in those with effort-related CAS or evidence of increased adrenergic tone before CAS-induced ischaemic attacks.5,17 In agreement with this view, some drugs known to reduce the central sympathetic drive, such as guanethidine and clonidine, have been reported to improve CAS in some patients with refractory variant angina.81
Conversely, α1-adrenoceptor antagonists have yielded inconsistent results in variant angina patients. Phenoxybenzamine and prazosin were reported to prevent CAS-related angina attacks in some small studies, but prazosin failed to achieve significant effects on angina attacks in other small studies.82–85 The development of pharmacological tolerance probably reduces the possible effects of this class of drugs.
Importantly, suppression of a heightened sympathetic drive to the heart has also been achieved with non-pharmacological ‘neural therapies’, including left stellate ganglion blockade, obtained with the use of anaesthetic drugs or radiofrequency ablation; endoscopic thoracic left sympathetic denervation; or even cardiac sympathetic denervation (plexectomy), usually applied in patients who have also undergone coronary artery bypass grafting (CABG).86–91
Coronary Intervention
A percutaneous coronary intervention (PCI) with stent implantation at the site of CAS, even in the absence of any significant stenosis, has been reported to facilitate symptom control and drug efficacy in patients with refractory CAS.92 However, CAS recurred proximally or distally to stent implantation in most patients; furthermore, the frequent multisite location of CAS in these patients does not usually result in satisfactory effects of this treatment.93 The indication for PCI seems reasonable in the presence of an organic obstructive stenosis, although CAS may also recur at the site of stent implantation in these patients.94 Similar issues apply to the use of brachytherapy, which has been reported to be effective in some case reports but may also itself facilitate CAS.95,96
Similar issues concern CABG, which has been used in some patients with refractory VSA.89–91 Notably, bypass of a vessel segment subjected to CAS in the absence of organic stenosis is associated with a high rate of graft occlusion and, therefore, should not be recommended in these patients.
Cardiac Device Implantation
Cardiac arrest caused by ventricular tachycardia/VF is the most severe complication of CAS-induced myocardial ischaemia. This dramatic event occurs only in a small, although appreciable, proportion of patients. The exact prevalence, however, remains poorly known and may depend on the characteristics of the population selected for investigation. Thus, a CAS-related cardiac arrest was documented in 7 of 202 (3.5%) Caucasian patients with typical variant angina, but in 35 of 1,429 (2.4%) Japanese patients with VSA.2,97
Implantation of an automatic ICD in patients with VSA who had a cardiac arrest or syncope caused by ventricular tachyarrhythmias during CAS-induced myocardial ischaemia is a controversial issue. The recurrence of cardiac arrest/syncope caused by ventricular tachyarrhythmias after a single episode of cardiac arrest is unlikely in patients in whom both angina symptoms and ischaemic episodes, as well as CAS induced by provocation tests, are fully prevented by optimal medical therapy.97–100 Thus, the indication for an ICD in these patients is questionable when also considering the potential issues related to device implantation, including the risk of inappropriate discharge and infection. Conversely, in patients in whom cardiac arrest occurs due to ventricular tachycardia/VF in the context of periods of refractory CAS or with CAS not well controlled by medical therapy (as indicated by the induction of CAS by provocation tests under optimal medical therapy), an ICD can be life saving and should therefore be implanted.
Similar considerations may apply to pacemaker implantation in patients who develop CAS-related syncope due to the development of severe bradyarrhythmias (atrioventricular block, sinoatrial disorders) during CAS-related episodes of transmural ischaemia.101 











Comments