Despite substantial advancements in rhythm control strategies during the last decade, AF remains a daily clinical problem with a steadily growing socio-economic burden worldwide.1 In the last few years, long-term success rates of ablation procedures have stood between 60% and 80% for paroxysmal AF (PxAF) and between 50% and 60% for persistent AF (PersAF), depending on the ablation strategy.2 Current international consensus on AF ablation has established the minimal acceptable 12-month AF-free rate off antiarrhythmic drugs after PersAF ablation at 40%.2
Catheter-based pulmonary vein isolation (PVI) is the standard invasive approach for AF ablation. However, the optimal ablation strategy beyond pulmonary vein isolation (PVI) for PersAF remains unclear.3 A wide variety of adjunctive ablation strategies have been explored, such as linear ablation, posterior wall isolation, ganglionic plexus ablation, isolation of the left atrial appendage, or substrate modification of areas with low voltage and fibrosis among others.4–9 While incremental benefit has been reported with some of these approaches, other studies have failed to show any advantage. The latter is probably related to the intrinsic limitations of anatomical approaches, which pay scant attention to the underlying mechanisms of complex wave propagation dynamics during AF. This lack of incremental benefit with additional anatomical-based ablation strategies goes against a classically accepted hypothesis of random propagation dynamics and non-hierarchical organisation in human PersAF.10
Recently, other approaches aimed at localising and targeting AF sources have led to the recognition of specific spatio-temporal patterns underlying what apparently turns out to be a chaotic and unstable rhythm.11 Experimental studies have demonstrated that AF can be sustained by high frequency drivers or sources, which can potentially be targeted to terminate the arrhythmia.12–14 During the last few years, novel mapping approaches have been implemented in the clinic aiming to identify AF drivers in complex PersAF cases. These mechanistic-based approaches have the potential to enable translational insights from experimental animal models into further improved specificity of ablation procedures and long-term freedom from AF (Table 1).15,16
Here, we summarise the state-of-the-art of mapping methods to identify AF drivers and potentially improve ablation outcomes. We review the main available clinical data facing the main controversies and possible solutions towards near-future directions, in the quest to increase the specificity of ablation strategies and a more personalised AF treatment.
Experimental Bases and Clinical Tools to Identify Drivers of AF
To date, a wide variety of methodological approaches have been used to identify AF drivers with the aim of increasing AF specificity in catheter-based ablation procedures, especially in complex clinical cases with recurrences despite PVI.11 Although the high spatio-temporal resolution of optical mapping would be the ideal scenario to map wave propagation dynamics during AF, in vivo optical mapping is still at early development stages in experimental settings, and we are still far from achieving further technological advances for clinical application.17 However, other approaches using novel hardware and software tools, unipolar or bipolar electrograms, sequential or simultaneous electrogram acquisitions have been developed to decrease the gap between the gold-standard optical mapping and current clinically available technology.
Optical Mapping and Phase Mapping to Identify Drivers of AF
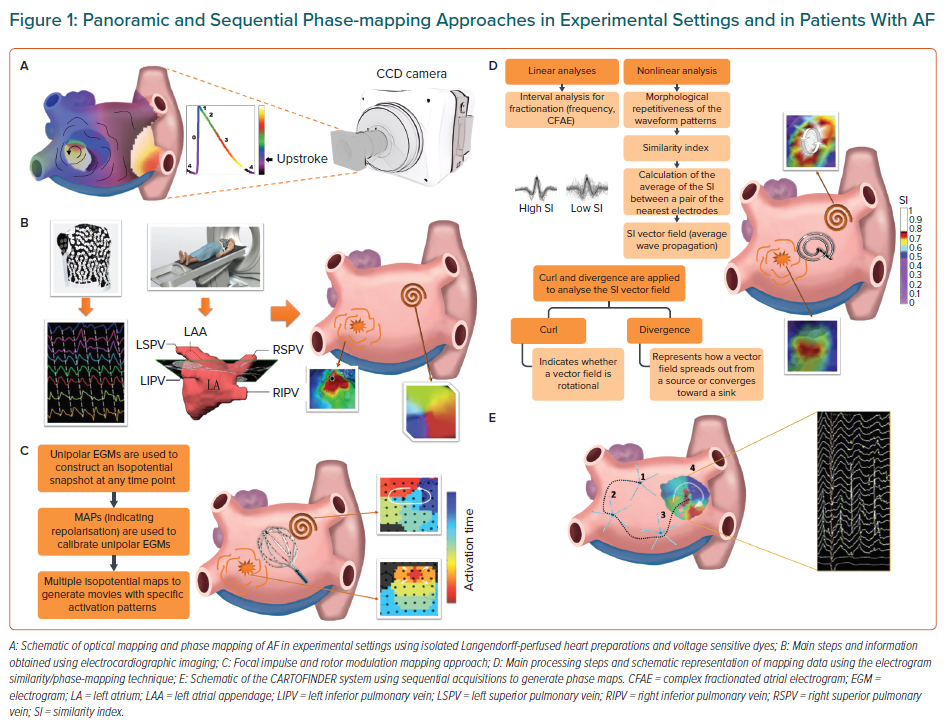
The advent of ex-vivo high-resolution optical mapping techniques has enabled visualisation of complex propagation dynamics with submillimeter resolution on simultaneously mapped large myocardial areas.18 This experimental mapping technology represents the current gold standard technique to study cardiac electrophysiology in whole heart preparations.17,18 In particular, the use of voltage-sensitive probes and high-resolution video imaging in isolated animal hearts has provided support for the hypothesis that AF may be driven by a single or a few high-frequency functional reentrant sources called rotors.12,19,20 Leading rotors can give rise to daughter rotational patterns and produce fibrillatory conduction by waves breaking against refractory barriers.21 Rotational drivers that maintain AF have been described as those with the highest frequency of rotation and whose domain of 1:1 activation is the narrowest.22
Rotors can be identified by the presence of scroll waves that span the atrial wall with different shapes of their 3D rotational axis (i.e. filament). In 2D, the most commonly described linear I-shape filament is on the epicardial or endocardial surface as a wave of excitation propagating around a point of uncertain phase called a phase singularity (the tip of the filament).23 The dynamics of the phase singularity may be determined by phase mapping the activation/recovery cycle (i.e., the action potential) at each time point (Figure 1A). In a phase map of functional reentry all the phases of the action potential converge at the phase singularity with a continuous progression of phase from −π to +π.24 Thus, the phase singularity is the pivot of the rotating pattern and its trajectory may circulate continuously around a small central region forming a circle (the core).20 The phase singularity trajectory may also meander (precess) around the same spot, or even drift never returning to the same point.24,25
Phase mapping forms the basis of many wave propagation analyses to identify phase singularities and rotational activity in current mechanistically based mapping methodologies used in clinical electrophysiology.26 Those studies have adapted the Hilbert transformation as a practical way to derive the signal phase from the voltage-time series, and to demonstrate rotational drivers underlying AF maintenance in PxAF, PersAF and long-lasting PersAF.26–28 In 80% of cases with non-PxAF, the drivers have been identified as rotors, which often colocalised with focal breakthroughs.15,16,29 Since then, several non-invasive (electrocardiographic imaging; ECGI) and invasive (focal impulse and rotor modulation [FIRM] and electrogram similarity/phase-mapping combined techniques) mapping approaches have used phase-mapping to guide driver detection and ablation (Tables 1 and 2, Figure 1).15,29,30
Electrocardiographic Imaging
Electrocardiographic imaging is based on a vectorial body surface representation of the cardiac electric field.31 This technology enables to investigate non-invasively different patterns of cardiac activation that may be relevant for the understanding of AF dynamics.32–34 Electrical signals from the patients’ torso are acquired using a high-density multielectrode array applied to the chest wall via a wearable vest. These signals are processed using sophisticated computer back-extrapolation (inverse solution) to deduce electrical activity on the heart surface.35 Both rotors and focal sources can be observed using ECGI (Figure 1B).36 Moreover, the complexity of wave propagation patterns increases with the duration of AF history, which is consistent with the expected effects of atrial remodelling.
Body surface potentials face significant challenges during complex arrhythmia such as AF. The potential field diminishes with distance from the epicardium to the body surface, which becomes problematic in the case of atrial signals, especially during wave propagation patterns like fibrillation.33,37 This results in low signal-to-noise ratios for recordings on the body surface, which may diffuse the interpretation of underlying atrial sources.33 The limitations of body surface recordings are further compounded when inverse solutions with regularisation methods are applied to minimise the effects of small errors in data collection (measurement noise, geometry errors, inaccurate conductivity values) that would otherwise cause significant errors in the solution.34 Despite these challenges, plausible source localisation has been obtained (Table 2), albeit with drawbacks associated with limited spatial resolution or the inability to distinguish endocardial from epicardial activation patterns.15
Focal Impulse and Rotor Modulation
Narayan et al. pioneered the clinical identification of rotational and focal impulses as a potential target to increase specificity in AF ablation procedures.38 The approach was based on experimental data in isolated animal hearts reporting that AF can be sustained by a few or small number of rotational drivers, which can also be observed as focal activity when the 3D filament is parallel to the endocardial or epicardial wall.39 Narayan et al. used simultaneous unipolar recordings from multielectrode basket catheters positioned in the left and right atrial cavities.38 Despite the low spatial resolution of the basket catheters to resolve the rotor core at the tip of a spiral wave, the authors calculated that based on knowledge of the human action potential duration and conduction velocity restitution curves, the minimal wavelength for reentry is 4–5 cm, which would be sufficient to resolve the rotating arms of the spiral wave emanating from the core of a rotor (Figure 1C).40–42
Narayan and co-authors enrolled 92 patients (107 consecutive ablation procedures) with highly symptomatic PxAF or PersAF in a two-arm 1:2 design with ablation of AF sources (FIRM-guided) followed by conventional ablation (mainly PVI; n=36), or conventional ablation alone (n=71; FIRM-blinded; Table 1).29 The data showed that FIRM ablation at patient-specific sources could acutely terminate or slow the AF cycle length and improve clinical outcomes. Although the initial results and long-term follow-up of this trial were promising, subsequent studies have shown more controversial results.43 Together with a high operator-dependent variability and technical limitations, the fact that active and passive sources cannot be discriminated by this technology might play an additional role regarding the controversial efficacy of AF driver elimination during FIRM-guided ablation.11,44
Similarity Index and Phase-mapping Combination
AF electrograms are characterised by wide temporal and spatial disparities, which make it difficult to visually identify any organised wave propagation patterns in real time. Complex fractionated atrial electrograms (CFAE) have been proposed to be associated with the surrounding of rotational activity.45 However, CFAEs have been proven to be highly unspecific, especially when using bipolar recordings, because multiple functional and structural irregularities in the atria can lead to CFAEs that are irrelevant to AF mechanisms.46–48 Such a lack of specificity has resulted in no incremental benefit when CFAEs are ablated after conventional PVI.49 Moreover, CFAE ablation may increase the risk of atypical atrial flutter due to lesion-related structural borders that may generate the substrate for other atrial arrhythmias.50
Lin et al. have proposed to improve CFAEs analysis using nonlinear-based waveform similarity analysis to identify the morphological repetitiveness of waveform patterns in fractionated electrograms (termed similarity index).51 The similarity index distribution can be displayed on the 3D electroanatomical mapping system in real time as similarity index vector fields, which can be obtained from the values between pairs of the nearest electrodes to show the average wave propagation (Table 2, Figure 1D). The analysis also enables to identify different wave propagation patterns including rotational activity.30 Lin et al. tested the clinical value of a combined methodology, including similarity index and phase mapping, in a prospective randomised trial that enrolled 68 patients with PersAF undergoing substrate modification after PVI (Table 1). AF had not terminated after PVI in any of the patients enrolled in the study. Patients were randomly allocated to group 1, undergoing similarity index and phase mapping-guided ablation, and group 2 receiving conventional CFAE ablation.30
In group 1, the authors identified an average of 2.6 ± 0.89 similarity index regions per chamber (rotors and focal sources in 65% and 77% of patients, respectively). Patients allocated to group 1 showed higher termination rates and lower AF recurrences than patients in group 2. Among potential limitations, it is worth mentioning that the interelectrode size of different catheters might have affected the bipolar recordings used for analysis. Moreover, sequential mapping might have affected the options to detect rotors compared with simultaneous mapping.38
Spectral Analysis and Advanced Electrogram Processing for Mapping AF
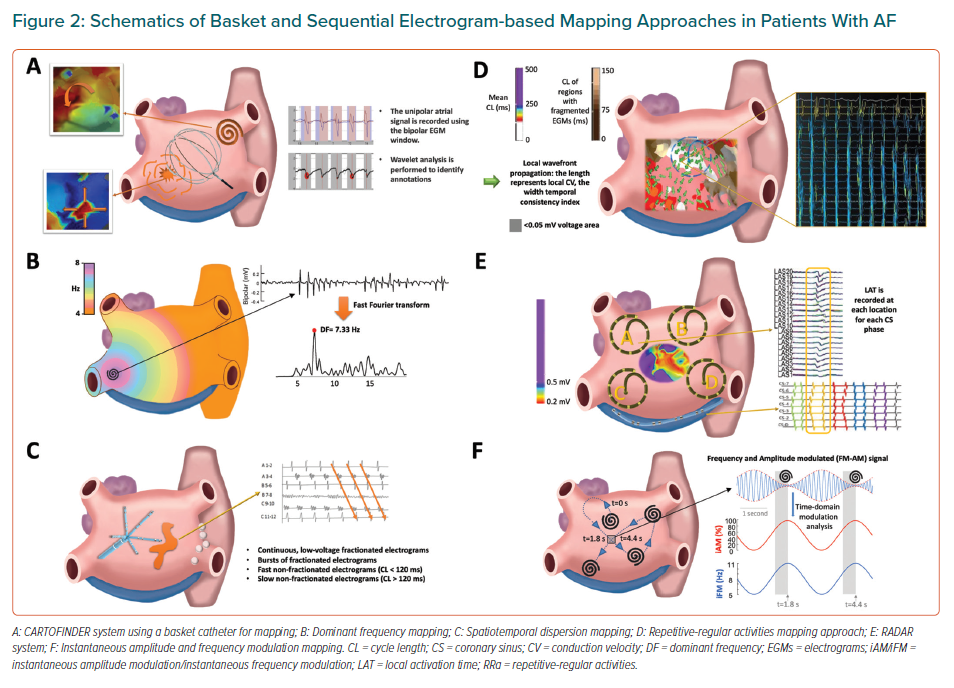
The use of phase mapping to identify the underlying dominant atrial region sustaining the overall fibrillation dynamics is subject to intrinsic limitations. Low spatial resolution of electrical recordings during clinical procedures makes interpolation of phases inherently biased toward rotor detection, since phase mapping algorithms are mainly devised to show rotational activity.29,52 Other mapping alternatives using spectral analysis of electrical recordings or advanced signal processing of intracardiac electrograms have been proposed to identify regions with specific temporal characteristics associated with AF drivers (Figures 2 and 3).
The CARTOFINDER
Recently, a novel module of the Carto System (Biosense Webster) called CARTOFINDER has been developed to map potential AF drivers through identifying repetitive activation patterns either focal or reentrant.53–55 Sequential and panoramic mapping can be achieved using a PentaRay catheter and a 64-pole-basket catheter, respectively (Figure 1E and Figure 2A), to acquire 30-second segments of unipolar signals at different atrial positions.53–55 Signal processing using the CARTOFINDER tool results in wavefront propagation maps (with or without associated frequency analysis) in an open format, where location points and electrograms can be further reviewed by the operator (Table 2).53,55
In particular, for each unipolar signal, two bipolar signals are created by pairing the electrode with the nearest two electrodes. A bipolar electrogram window is then applied to the unipolar signals that range from earliest onset to the latest offset of the two bipolar electrograms. Atrial signals within the bipolar electrogram window are then annotated, whereas areas outside this window are excluded. Atrial signals are annotated at the point of the maximum negative unipolar slope (peak negative dv/dt) using wavelet analysis to determine local activation time.
In patients with PersAF (average AF duration 13.5 ± 6.2 months) undergoing AF ablation, Honarbakhsh et al. have described transient but recurrent rotational activity in approximately two-thirds of instances, mostly confined to low-voltage zones.53 The remaining one-third of focal activity did not show any predilection for low-voltage zones. CARTOFINDER-guided AF driver ablation yielded a positive response (in terms of AF termination or cycle length slowing ≥30 ms) in 84.6% of cases, with at least one driver with a positive response to ablation in all patients.53 More specifically, Honarbakhsh et al. described AF termination in 12 of 19 patients after the ablation of driver sites. Although AF termination rate may be considered an endpoint of acute procedural success, recent data have questioned its potential to predict freedom from AF in the long-term follow-up and should therefore be considered with caution.2,56 This acute ablation success to terminate AF was much lower in a previous study by Calvo et al., who also used the CARTOFINDER system in patients with long-standing PersAF (Table 1). In this more complex setting, AF termination was only achieved in two of 13 patients after targeting rotor domains, which were defined as areas with rotational activity lasting for ≥three consecutive rotations and with repetitive patterns over a 30-s acquisition period.55
Spectral Analysis and Dominant Frequency Mapping
Spectral analysis and dominant frequency (DF) have been proposed as a robust method to identify AF driver regions associated with hierarchically activated atrial domains.12,57 Irrespective of the predominant wave propagation patterns, either rotors or focal activity, spectral analysis can identify the regions with the fastest frequency domain, which likely maintain the overall arrhythmia. Although this activation rate hierarchy is particularly evident in PxAF, in more complex scenarios like PersAF, atrial frequency domains tend to be more homogeneous and difficult to distinguish.57,58 The latter complicates the identification of a single atrial region that might correspond to the dominant AF driving source. In fact, in advanced remodelling stages, it is common to observe more than one atrial region with high-frequency AF drivers.59
The most common approach to identify atrial regions with the fastest activation rates (i.e. the shortest cycle lengths) is to perform spectral analysis to identify the DF. Briefly, the spectrum of a signal displays its energy/power distribution in the frequency domain. The frequency of the highest peak in the spectrum at each spatial location is called DF (Figure 2B), which is often used as a surrogate for the average activation rate (i.e. the inverse of the cycle length) at that location.60 Spectral analysis becomes particularly useful when the activation rate is difficult to measure in the time domain, as may be the case with fragmented signals during AF.37 However, DF analysis is sensitive to bias when using a fully automatic approach that can be affected by harmonic peaks.61 Moreover, the more complex the signal is the more complex the spectrum will be. Thus, spectral peaks with similar power may make it difficult to select the peak associated with the underlying intrinsic activation rate of a specific atrial spot. Some of these limitations may have affected the results of the multicentre randomised clinical trial RADAR-AF (Table 1). In PersAF patients, the study reported no incremental benefit on freedom from AF at 6 months of follow-up after PVI plus DF-guided high-frequency source ablation compared with PVI alone.62 In addition, the RADAR-AF trial used the analysis of bipolar signals (Table 2), which are more prone to be fractionated with more complex spectra compared with unipolar recordings.63
Spatio-temporal Dispersion of Electrograms
Another approach involving the analysis of fractionated electrograms has suggested that fractionation occurring in a non-simultaneous fashion at neighbouring electrode locations (time dispersion) and organised in well-defined clusters (spatial dispersion) may indicate the presence of an underlying source of AF (Tables 1 and 2).64 As described by Seitz et al., the approach takes advantage of using a traditional high-resolution electroanatomic mapping obtained with a multielectrode catheter, sequentially positioned in various atrial regions for a minimum acquisition time-period of 2.5 seconds. Then, the data are visually analysed to identify atrial locations displaying spatiotemporal dispersion on bipolar electrograms. Areas with dispersion have been defined as clusters of electrograms, either fractionated or non-fractionated, that display inter-electrode time and space dispersion at a minimum of three adjacent bipoles such that activation spreads over all the AF cycle length (Figure 2C).64
Seitz et al. tested this approach in a prospective study including 105 patients undergoing AF ablation (non-PxAF; 77.2%). The authors used a Pentaray catheter to map under electroanatomical guidance with the Carto system. The ablation was performed only in regions displaying electrogram dispersion criteria during AF, which terminated (mainly initial conversion to atrial tachycardia; 85%) in 95% of patients. These results were obtained after the ablation of 7.5 ± 2.5% (10.1 ± 4% of the left atrial surface) and 15 ± 4% of the total atrial surface (29 ± 9.7% of the left atrial surface) in PersAF and long-standing PersAF, respectively. After 18 months of follow-up, atrial arrhythmia recurrences were reported in 15% of patients. These results were complemented with 2D computational simulations and optical mapping data from ovine atrial scar-related AF. In this experimental setting, pseudomultipolar electrograms exhibiting dispersion were specifically recorded in the vicinity of rotors. Moreover, dispersion was larger in the presence of interstitial fibrosis.64 Although encouraging, these results will need to be confirmed by other groups. Ablation of large atrial areas (up to 30% of the left atrial surface) may reflect a potential lack of specificity. Moreover, using visual inspection to identify areas with spatio-temporal dispersion of electrograms makes it difficult to follow an objective criterion for ablation. Map stability and reproducibility of target sites have not been addressed either, which raises the concern of potential significant variability on target sites as may happen for CFAE regions.65
Repetitive-regular Activities
Other alternatives may provide novel options to identify areas with fast atrial activation rates and unravel the hierarchical patterns of complex AF dynamics. On this line, Pappone et al. have proposed a new method based on cycle length analysis, variability of electrograms and conduction velocity vectors to identify the direction of wavefront propagation, the speed of the repetitive-regular activations and the fractionation index, which is measured by an automatic algorithm to show the regions exhibiting fragmented electrograms (Table 1, Figure 2D).66 The regions showing repetitive and regular activations with a mean cycle length ≤220 ms and a cycle length standard deviation between 0 and 30 ms were considered as ablation targets. These regions showed distinct peak–peak activations, small variation in activation cycle length, consistent electrogram morphology, flat isoelectric interval between consecutive activations and consistent activation sequences on the multielectrode mapping catheter over time. The methodology excluded irregular activations (>30 ms variability), fractionation (60–120 ms cycle length) and regions with voltage <0.05 mV from the analysis to identify AF sources (Table 2).66
The approach of Pappone et al. was tested in a prospective, single-centre randomised trial, in which PersAF patients undergoing AF ablation were randomly allocated to ablation of repetitive-regular activations followed by PVI (mapping group; n=41) or PVI alone (control group; n=40). The regions exhibiting repetitive-regular activities were 479 in 81 patients (5.9 ± 2.4 per patient), and 39% of repetitive regular activities were identified within the PVs. Ablation of repetitive-regular activities resulted in higher arrhythmia termination rates compared with conventional PVI (61% versus 30%). After 1 year of follow-up, 73.2% of patients in the mapping group were free from AF recurrences versus 50% in the control group.66
The mapping criteria excluded irregular yet repetitive electrograms patterns and fast temporal activations, which might also have potential mechanistic value. This could have led to missing relevant AF driver regions, which may have affected the sensitivity to detect AF sources. The authors did not map the right atrium either, which may also have affected study outcomes in this cohort of patients with PersAF and more complex substrates.67
New Approaches to Improve Spatial Resolution During AF Mapping
Spatial resolution represents a significant problem during AF mapping, specially using simultaneous mapping with low-density multipolar catheters. This limitation makes it difficult to identify AF drivers in real time.29 Moreover, most rotational activity associated with driver regions has been described with drifting behaviour in experimental settings using high-resolution optical mapping, and only occasionally a driver can be recorded remaining stable in the same position for long recording periods.12,13,19 Therefore, limited spatial resolution in the clinic may cause rotors to be missed, improperly localised, or artefactually created due to highly interpolated maps.68,69 Two innovative systems have been recently developed to potentially overcome these technical limitations and provide real-time and more panoramic insights.
Real-time Electrogram Analysis for Drivers of AF
The Real-time Electrogram Analysis for Drivers of Atrial Fibrillation (RADAR) system (AFTx, Inc.) uses high-density electroanatomical maps, created with standard mapping equipment and unipolar signals.70 Local unipolar atrial electrograms and electrode positions are extracted from the mapping system and matched to the corresponding simultaneous coronary sinus pattern, or phase (not to be confused with the aforementioned phase mapping approach), which is used as a reference. Electrograms from different anatomical locations are processed to create a high-resolution panoramic 3D conduction vector map for each coronary sinus phase. This enables to calculate conduction vectors using the distance between electrodes on the mapping system and the depolarisation timings at each electrode. Thus, the system identifies sites with spatiotemporal repetition of drivers and generates driver density maps, which are the result of analysing individual vector maps (Table 2, Figure 2E). Areas of voltage transition can also be identified to specifically highlight rotational or focal activity localised in these areas. To date, clinical outcomes reported with this system mainly rely on a clinical series conducted by Choudry et al.70 The study included 64 patients with PersAF or long-standing PersAF undergoing AF ablation with the RADAR system. After PVI, the system was used to target and ablate all driver domains. If the patient remained in AF after ablation of all driver domains, additional RADAR maps could be created in the left or right atrium at the discretion of the operator. The authors reported acute AF termination in 55% of patients (Table 1). After 1-year follow-up 68% remained free of AF off all antiarrhythmic drugs.70
Non-contact Charge Density Mapping
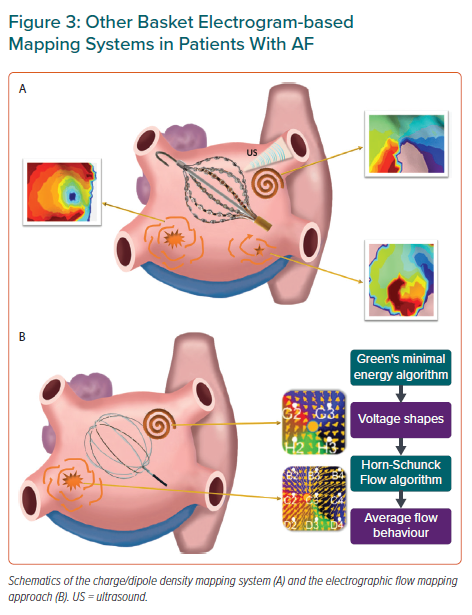
Complex wave propagation patterns as those documented during AF may require simultaneous or very rapid high-resolution mapping of both atrial chambers to identify relevant regions sustaining the overall arrhythmia.12,71,72 This would be crucial if fibrillation dynamics were completely random and driver regions were not stable during sequential mapping. A novel mapping approach has been proposed to address this potential limitation of conventional sequential mapping in the clinic. The method uses a combination of ultrasound-based real time reconstruction of the endocardial surface with simultaneous acquisition of intracardiac unipolar voltage. Inverse and forward algorithms are then applied to the intracardiac voltage to obtain the distribution of positive and negative ionic charges and display electrical activation on the generated anatomical shell (Figure 3A).73,74 The maps obtained with this method provide an average fourfold improvement in spatial and temporal resolution compared with conventional voltage maps. This relies not only on the panoramic acquisition of the electrode positions but also on the integration of high-resolution real-time anatomical data of the ultrasound transducers, which minimise errors due to motion, and provide accurate distance measurements for overall system stability.74
The UNCOVER-AF trial has recently confirmed the feasibility and safety of this novel system during AF ablation (Tables 1 and 2).75 Thirteen centres across Europe and Canada prospectively enrolled 129 patients with PersAF undergoing de novo catheter ablation combining PVI + charge density-guided ablation. The main results of the study showed that after 1-year follow-up a single procedure achieved 72.5% freedom from AF on or off antiarrhythmic drugs. The primary safety outcome was 98% with no device-related major adverse events.
In Search of Greater Specificity in the AF Ablation Strategy
Targeting passive atrial structures with catheter ablation might result in unnecessary scar formation, potentially increasing the likelihood of iatrogenic atrial tachyarrhythmias, loss of contractile function and other sequelae without a significant impact on long-term ablation success.76 Interesting novelties in the field may improve characterisation of AF drivers and decrease unnecessary ablation sites.
Electrographic Flow Mapping
This novel technology provides the ability for a full spatial and temporal reconstruction of electrographic potentials and their flow, derived from endocardial unipolar electrogram data collected with a 64-pole basket catheter.77 Electrographic flow mapping designates the matrix of velocity vectors describing the average propagation of action potentials by acquisition of unipolar recordings (Table 2, Figure 3B). The system is not affected by limitations of spatial resolution due to the algorithm providing triangulation between the electrodes as long as electrode distance is larger than the excitation wavelength.
Each electrographic flow map is automatically analysed to discriminate between active and passive drivers. Active drivers are characterised by vectors spreading away from an AF source. Conversely, passive drivers show flow phenomena around a distinct area with the vectors running towards this area. Clinical data have shown that electrographic flow mapping was able to identify the majority of AF sources detected with FIRM, although 40% of them were classified as passive flow phenomena (Table 1).77 Therefore, electrographic flow mapping may potentially increase specificity on ablating AF drivers although validation warrants further confirmation in dedicated series.
The Instantaneous Amplitude Modulation and Instantaneous Frequency Modulation Mapping Algorithm
Quintanilla et al. have recently proposed a new algorithm capable of distinguishing the dominant drivers in AF, which may also increase the specificity of AF ablation in complex PersAF cases (Table 1).63 Based on the assumption that the amplitude and frequency modulations used for radio broadcasting are naturally present in signals during cardiac fibrillation,78 the combined analysis of the instantaneous frequency modulation (iFM) and instantaneous amplitude modulation (iAM) of single-signals (optical action potentials or unipolar atrial signals) during PersAF could detect AF drivers and display their hierarchical organisation without costly panoramic multielectrode acquisition systems (Table 2 and Figure 2F). The authors used a completely translational approach, including computer simulations, optical mapping of isolated Langendorff-perfused sheep hearts with PersAF, in vivo electroanatomical mapping of a clinically-relevant porcine model of long-term self-sustained PersAF, and complementary studies in patients with PersAF.
The results showed that both rotational-footprint and spatiotemporally stable leading-driver regions can be identified using the iFM/iAM algorithm without panoramic multielectrode acquisition systems.
In the pig model of long-term PersAF, the arrhythmia terminated during the ablation of leading-driver regions in 92.3% ablation procedures after a median of 16.9 minutes of radiofrequency delivery. Importantly, despite the fact that all the leading-driver regions displayed rotational-footprints, they only constituted around a quarter of total rotational-footprint positive locations. This demonstrates that rotational activations are sensitive but not specific to guide AF ablation. All identified leading-driver regions were targeted in two of the three patients of the interventional group, with subsequent freedom from AF recurrence after 16 months of follow-up off antiarrhythmic drugs.63
Unlike phase mapping, which requires multiple signals to detect rotations,79 the iFM/iAM algorithm detects rotational-footprints for each signal independently. Also different from DF mapping, iFM tracks dynamic changes in the local activation rate during acquisition, enabling the detection of intervals with rotational-footprint or high-frequency short bursts that would be easily missed by DF analysis.63
Furthermore, this new algorithm relies on unipolar signals from 1 mm electrodes after advanced signal processing and careful ventricular far-field minimisation/rejection, instead of bipolar signals whose amplitudes depend on wavefront orientation. Although promising, this novel algorithm needs further validation in larger sets, also to quantify its potential synergistic value with respect to PVI.
Main Clinical Evidence Using Novel Mapping Methods For AF Ablation
The steady increase in the number of mapping techniques to identify and ablate potential AF drivers has raised the need to assess their effectiveness and safety in large clinical trials, either if performed as a complementary step after PVI (e.g. in PersAF cases) or instead of PVI (e.g. in PxAF cases).
Currently, the main clinical evidence comes from the pioneering approach of Narayan et al. using FIRM.16,38 Initial meta-analyses focused on studies comparing PVI alone and PVI plus FIRM ablation. In particular, Parameswaran et al. included 11 observational studies (four studies with PxAF patients and 10 with PersAF patients) with a total of 556 patients (166 PxAF and 390 PersAF patients).80 The data showed wide variation in reported efficacy, which could not be explained by clear differences between the studies. Single procedure freedom from AF was 37.8% for PxAF and 59.2% for PersAF at a mean follow-up of 13.8 and 12.9 months, respectively. These data suggest the possibility that FIRM-guided ablation may have pro-arrhythmic effects in PxAF.
Another meta-analysis by Mohanty et al. did not show any therapeutic benefit of PVI plus FIRM ablation compared with PVI alone.81 Both meta-analyses show limitations to report stronger results. Thus, the majority of studies included in the analyses were uncontrolled with a relatively small sample size (<200 patients) and marked heterogeneity and variability in success rates among different centres performing FIRM ablation. Such variability in results may reflect a complex procedure that requires additional skills for mapping, acquisition and interpretation before ablation.
Beyond the FIRM approach, other meta-analyses have supported the potential benefit of a combined strategy involving other phase-mapping and electrogram-based techniques with PVI in improving both acute AF termination rates and single-procedure freedom from AF. This can be achieved with similar rates of procedural complications as in conventional PVI.82,83 Remarkably, in controlled trials, the addition of AF driver ablation to PVI supports the possible benefit of this combined approach to improve AF ablation outcomes.
A step towards better understanding of the clinical impact of different AF driver mapping methodologies has recently been performed by Lin et al. in patients with PersAF.28 Their meta-analysis confirmed that adjunctive driver-guided ablation in addition to PVI could improve one-year AF freedom and increase acute AF termination rate without a significant increase in potential complications. The benefit in PersAF ablation seems to be more evident using phase-mapping methodologies rather than electrogram-based driver mapping.28
From the foregoing, although the data from meta-analyses provide some support to target and ablate driver regions plus PVI in PersAF patients, the evidence mainly comes from uncontrolled and non-randomised studies, with substantial heterogeneity in reported outcomes. In addition, the analyses are based on the premise that these mapping tools are adequate for detecting AF drivers. However, the considerable heterogeneity among AF driver mapping technologies precludes data analysis from drawing firm conclusions on driver-guided ablation.
Actually, only a few studies have compared different driver-based mapping approaches. These data show some agreement on target sites between phase mapping and other methods as electrographic flow mapping or a method that reconstructs AF signals using sinusoids in an attempt not to detect false rotational activations.84,85 However, to the best of our knowledge, actual validation in experimental settings using optical mapping comparisons of the current wide range of methodologies has not been performed.
Data variability on reported outcomes may be affected by clinical and procedural differences, and among individual operators. In particular, evidence against the effectiveness of any specific driver-guided AF ablation strategy need not undermine its validity, but may instead reflect some shortcomings of the technology or even differences in atrial debulking among operators.
Overall, evidence for the efficacy of AF driver ablation remains inconclusive. Further prospective randomised studies with standardised driver identification and validation are warranted.
Conclusion
Rotors/focal sources are an important mechanism for sustaining AF in animal models and they are increasingly being demonstrated in human AF by a variety of mapping approaches. However, other mechanisms may certainly be involved in initiation and maintenance of specific cases.86 Recent studies using different novel mapping approaches to identify, target and ablate potential AF sources have been reported with promising results.
Such mechanistic-based ablation techniques have evolved thanks to insights into wave propagation dynamics during AF provided by experimental optical mapping studies and computer-based simulations. Still, current mechanistic-based ablation strategies have provided conflicting results, often linked to technical limitations of the methodology and also partial knowledge of AF pathophysiology.
In the near future, the development of more specific and reliable ablation methods is an important goal to improve overall safety and clinical outcomes of AF ablation. This will help to decrease the substrate for subsequent development of macroreentrant atrial arrhythmias and minimise the extent of atrial ablation so as to preserve atrial contraction.
Clinical Perspective
- Current long-term success rates after catheter ablation of persistent AF are not higher than 60%, highlighting the need for more effective strategies.
- A wide variety of adjunctive anatomical ablation strategies have been explored, without convincing evidence of benefit and with potential risk of pro-arrhythmia and higher complication rates.
- Developments in mapping tools and computational methods for advanced signal processing have provided novel strategies to identify atrial regions potentially associated with AF maintenance.
- These novel mapping tools represent a significant step forward towards the understanding of complex patterns of propagation during AF and the potential achievement of patient-tailored ablation strategies in the near future.