The supply of oxygen is an essential requirement for adequate myocardial metabolism, and it is regulated by a continuous adjustment of coronary blood flow according to the actual demand. The processes involved in coronary vasomotion are complex and encompass vasodilative and vasoconstrictive properties. Functional impairments of these mechanisms are possible causes of angina with non-obstructed coronary arteries (ANOCA).1,2 In order to assess coronary vasomotion, non-invasive and invasive techniques have been developed during the last decades. Non-invasive techniques have recently been reviewed.3 They have the advantage of avoiding the small, yet definite, risk associated with an invasive procedure. Non-invasive techniques for testing abnormalities of coronary constriction (spasm testing), however, have several important limitations:
- To assess coronary spasm, only ergonovine (ER) can be given IV as a provocation substance due to the short half-life of acetylcholine (ACh).
- Given that the vasomotion of a single coronary vessel cannot be tested, IV ER may lead to multivessel spasm. In this case counteracting medications can be given only sublingually or by IV injection, which may be insufficient to resolve prolonged spasm.4
- They do not allow discrimination between focal and diffuse epicardial spasm or between microvascular and epicardial spasm.
- Detection of spasm depends on the recognition of regional perfusion defects or wall motion abnormalities by (contrast) echocardiography, which may be difficult in case of suboptimal acoustic windows.5,6
Given that invasive tests can be easily applied to patients undergoing diagnostic coronary angiography, this review shall summarise currently well-established invasive methods for the diagnosis of coronary functional disorders.
Methods for the Assessment of Coronary Functional Disorders
Coronary vasomotion is the summed effect of a highly sophisticated interplay between vasoconstrictive and vasodilative actions. Several tests addressing different mechanisms of coronary vasomotion need to be applied for a comprehensive assessment. The type and sequence of tests currently differs from centre to centre depending on the equipment and expertise available in the respective medical centre.
Assessment of Coronary Vasodilatation
The main mechanism of increasing coronary blood flow is the dilatation of microvascular resistance vessels, which can be stimulated by an increase in the metabolic activity of the heart. Hence, in the healthy heart, myocardial metabolism (demand) and coronary blood flow (supply) are always matched.7 This vasodilative process (also called coronary autoregulation), however, has been shown to be impaired in various forms of heart disease. Reduced vasodilatation may lead to angina when oxygen demand is increased, for instance during exercise. The effect is very similar to the situation in which an adequate increase in coronary blood flow is restricted by obstructive lesions in epicardial coronary arteries.8 In order to prove that inadequate vasodilatation produces angina in patients without epicardial obstructive disease, coronary blood flow velocity is measured both at rest and during maximal vasodilatation (hyperaemia). For the latter, IV or intracoronary injections of adenosine are usually used due to its favourable short half-life compared with alternative drugs such as dipyridamole or papaverine.9 Coronary blood flow velocity can be determined by two different techniques.
Direct Measurement of Flow Velocity using the Doppler Shift
The Doppler shift has been used since the 1970s to determine coronary blood flow velocity as the basis for further calculations of coronary flow reserve (CFR) and coronary resistance.10 A Doppler wire is inserted into the coronary artery to be measured. A sonic wave of a defined transmitting frequency is sent from the tip of the Doppler wire, which is then reflected by the erythrocytes, which alter the wave’s frequency depending on the flow velocity of the erythrocytes.11 The main limitation of the technique is that it may be difficult to obtain an optimal Doppler signal.12,13 However, with careful repositioning it is possible to finally receive useful Doppler signals in most patients.14 Sometimes it may be necessary to stabilise the position of the Doppler wire using an intracoronary microcatheter. Despite these challenges and alternative techniques available, intracoronary Doppler measurements are still successfully used in daily clinical practice.3,15
Indirect Measurement of Flow Velocity using the Thermodilution Technique
The principle of the thermodilution technique for the calculation of coronary blood flow velocity was developed by de Bruyne and Pijls in 2001.16,17 A bolus of room-temperature saline is injected into the coronary artery. The distal microsensor can be used to measure both pressure and temperature. The proximal temperature is monitored using the shaft of the wire, which changes its electrical resistance depending on the surrounding temperature.16 Proximal and distal temperature data are used by dedicated software to determine the so-called transit time of the saline moving with the bloodstream. The measurement of three injections at room temperature are averaged to define the mean transit time (Tmn). This parameter has been shown to correlate inversely with the volumetric coronary flow measured in vitro (r=−0.75; p<0.001) and in vivo (R2=0.72).16,18 Intracoronary thermodilution is the most widely used technique to assess coronary flow today.19,20 However, thermodilution-based measurements are usually performed during an extended state of hyperaemia (>30 seconds).17 This steady-state hyperaemia is usually induced by IV adenosine, which is associated with more side-effects and patient discomfort than intracoronary injections.21 However, the intracoronary application of adenosine for thermodilution-based measurement has recently been described.22 Adenosine needs to be given first into the coronary artery and, following a lag time of a few seconds until steady-state hyperaemia has been reached, a bolus of saline is injected.23 Repeated injections of adenosine may be required for the three measurements during hyperaemia, and accurate timing is mandatory.22
Once coronary blood flow velocity has been measured it can be used to quantify the vasodilative potential of the coronary vasculature by the derivation of different parameters.
Coronary Flow Reserve
CFR, which reflects the relative increase of blood flow velocity during hyperaemia, has been the most widely used parameter for assessing the vasodilative potential of the coronary microvasculature. For Doppler-derived measurements, CFR is calculated as the ratio of the average coronary peak flow velocity during hyperaemia to the average flow velocity at rest. CFR can also be derived from thermodilution measurements. It is calculated inversely as the ratio of Tmn at rest to Tmn at hyperaemia, considering the negative correlation of Tmn and blood flow velocity.16 A reduced CFR is a predictor of major adverse cardiac events such as cardiovascular death, stroke, MI and heart failure hospitalisation.24 According to the current guidelines of the European Society of Cardiology (ESC) for the diagnosis and management of chronic coronary syndromes, an abnormal CFR is defined as <2.0.1
Does one of the techniques of CFR measurement have a clear advantage over the other with respect to the accuracy of the measurements? Doppler-derived CFR (CFRDoppler) and thermodilution-derived CFR (CFRThermo) correlate quite well.13,15–17,25 There are some data favouring CFRThermo over CFRDoppler. Fearon et al. compared both methods with an external flow probe (CFRFlow) and reported a stronger correlation between CFRThermo and CFRFlow (r=0.85; p<0.001) than between CFRDoppler and CFRFlow (r=0.72; p<0.001). Indeed, in that study CFRDoppler was observed to have a greater scattering of values.25 In contrast, Everaars et al. used [15O]H2O PET as the gold standard and found a significantly higher correlation between CFRDoppler and CFRPET (r=0.82; p<0.001) than between CFRThermo and CFRPET (r=0.55; p<0.001).12 In that study the scattering was higher for CFRThermo than for CFRDoppler. Thus, it is currently not clear which of the two measurement techniques is more reliable in determining true CFR.
However, CFRThermo tends to provide higher values than CFRDoppler.12,13,16,17,25 Thus, the correct cut-off value may depend on the technique chosen. Hence, it is likely that the absolute cut-off value of <2.0 as recommended by current guidelines is an oversimplification.1 This may result in a significant underestimation of the true prevalence of disease due to a low sensitivity and a low negative predictive value. The true range of normal values is difficult to determine because this would require intracoronary measurements in normal volunteers of different age groups. A study performed in patients shortly after heart transplantation using papaverine for maximal coronary vasodilatation noted mean values for CFRDoppler of 4.5 with a narrow standard deviation of 0.2.26 Hence, in that population of mostly young hearts the lower limit of normal was 4.1. The Coronary Vasomotion Disorders International Study group (COVADIS) acknowledged a range of CFR values of ≤2.0 to ≤2.5, depending on the method chosen, as possible cut-offs, but this is also likely a significant underestimation of the true normal range of reactions to vasodilator stimuli.27 Matters are even more complicated because myocardial perfusion reserve (and hence likely also CFR) is age dependent and decreases with advancing age.28 Moreover, coronary microvascular rarefaction regularly occurs later in life, further limiting the maximum delivery of oxygen to individual myocardial cells.29 Thus, it becomes obvious that the threshold at which symptoms related to myocardial ischaemia occur may vary from patient to patient.
The main disadvantage of CFR as a single parameter is that this parameter may reflect two pathophysiologically different conditions. It is commonly accepted and assumed that reduced CFR is indicative of a reduced maximal coronary flow velocity. However, coronary blood flow at rest is another major determinant of CFR. A reduced CFR is also frequently found in patients who have an increased resting flow velocity but who also have a preserved and almost normal maximal flow velocity following pharmacologic vasodilatation.30,31 This condition was also found in a porcine model of microvascular disease introduced by exposure to various coronary risk factors even before the development of atherosclerotic lesions in the epicardial arteries.32 Hence, a reduced CFR may reflect a limited vasodilative potential, despite a near normal maximal vasodilatation when the microvasculature is already dilated at rest, in response to conditions that make a higher blood flow at rest necessary.8,30
This limitation of CFR might be overcome by evaluating additional parameters.
Microvascular Resistance
Microvascular resistance is the ratio of distal coronary pressure to distal coronary flow velocity. If resistance is measured using thermodilution, it is assumed that distal coronary flow velocity is equal to proximal coronary flow velocity. An advantage of measuring microvascular resistance is that its value at maximal hyperaemia is independent of coronary blood flow at rest.33,34 In contrast, the assessment of hyperaemic microvascular resistance (HMR) only will not reflect the limited vasodilative capacity often seen in women with exercise-related symptoms in whom the major abnormality is the increased flow at rest.30
Hyperaemic Microvascular Resistance
HMR is determined by dividing distal coronary pressure, Pd, by Doppler-derived average peak flow velocity (APV).35 Pd is measured using the pressure sensor incorporated into the Doppler-equipped guidewire (ComboWire, Philips Volcano). Higher HMR values indicate higher microvascular resistance. There is currently no guideline-recommended threshold of HMR above which it should be considered abnormal.1 Williams et al. report the highest specificity and sensitivity in diagnosing microvascular dysfunction for a threshold of ≥2.5 as compared with the gold standard, a cardiac magnetic resonance imaging-derived myocardial perfusion index.15 This is in accordance with the findings of Van de Hoef et al., who measured HMR values of between 1.5 and 2.5 in a normal reference vessel (<30% obstruction) in patients with obstructive coronary artery disease (CAD) in another vessel.36
Index of Microcirculatory Resistance
Alternatively, the index of microcirculatory resistance (IMR), which is based on the thermodilution principle as described above, can be calculated by multiplying Pd by Tmn. IMR shows a good correlation (r=0.54; p<0.0001) with microvascular resistance as measured in a porcine model via an ultrasonic flow probe, using a pressure wire as the reference standard.37 As proposed by COVADIS and successfully applied in the CorMicA trial, a threshold value of ≥25, above which resistance is abnormally high, has made its way into current guideline recommendations.1,27,38,39 The threshold of 25 was derived from the IMR values of only 15 patients who had no clinical evidence of atherosclerosis on angiography.38 These patients underwent cardiac catheterisation for non-coronary reasons (prior to closure of a patent foramen ovale or electrophysiological examination for cardiac arrhythmia). These patients had never reported chest pain and had minimal risk factors for CAD. Mean IMR in the control group was 19 ± 5 with a range of 8–28. Hence, the currently used cut-off for abnormal IMR was derived from this mean value +1 SD. A different approach for defining cut-off values was reported in a recent paper from Japan.20 Suda et al. studied 187 patients who underwent ACh provocation testing and measurement of IMR to evaluate coronary microvascular function.20 All of these patients were followed for a median of 893 days. Major adverse cardiovascular events were defined as the composite of cardiac death, non-fatal MI and hospitalisation due to unstable angina. IMR was correlated with the incidence of cardiac events, and the optimal cut-off value was identified on receiver operating characteristic curve analysis as IMR ≥ 18. Events were more common when IMR was 18 or above, however, only 10 events occurred (cardiovascular death, n=1; hospitalisation for unstable angina, n=9). Therefore one needs to be aware of the limitations of currently available cut-offs for microvascular resistance measurements.
Thus, both methods of evaluating microvascular resistance correlate only modestly with each other (r=0.39; p=0.0006), probably due to the different approaches for determining blood flow velocity.15
Assessment of Endothelial Dysfunction
ACh causes vasodilatation of the epicardial vessels and the microvasculature in normal individuals unless high doses are applied, which may lead to some vasoconstriction but not to coronary spasm.40,41 This is because the vasodilative effect of low doses of ACh mediated by the healthy endothelium is more pronounced than the vasoconstrictive opposing effect of the substance on vascular smooth muscle cells. Dysfunctional endothelium will not be able to release nitric oxide in sufficient amounts and the vasoconstrictive effect caused by the direct action on the vascular smooth muscle cell will become more prominent, resulting in less vasodilatation.42 There are two well-established threshold values for diagnosing endothelial dysfunction. Following intracoronary injection of any dose of ACh, endothelial-dependent microvascular dysfunction is defined as an increase of coronary blood flow ≤50%, whereas a decrease in coronary artery diameter ≥20% indicates epicardial endothelial dysfunction.43 Endothelial dysfunction is associated with cardiac events such as cardiac death or percutaneous coronary revascularisation.44
In order to measure endothelial function, coronary blood flow is calculated by multiplying mean flow velocity (estimated as 0.5 × APV) by vessel cross-sectional area both at baseline and following the injection of ACh.11 Coronary diameters are usually determined 5 mm distal to the tip of the Doppler wire using quantitative coronary angiography.44
Protocols for the assessment of endothelial function vary between centres. In most reports, incremental ACh dosages between 0.4 and 55 µg are used, depending on the concentration of the injectate (0.18–30 µg/ml), the injection time (2–3 minutes) and the injection rate (unspecified or 0.5–1 ml/min).38,44–47
Ultimately, this form of coronary function testing reflects abnormalities of vasodilatation, although not as a response to adenosine, but to ACh. Thus, the vasodilative capacity of the microvasculature is measured using two different methods: ACh-based endothelial function testing and standard adenosine testing. It is, however, not clear whether these two different ways of assessing the vasodilative capacity of the microvasculature provide additive information to each other. Sara et al. defined coronary microvascular dysfunction either as a reduction in CFR in response to adenosine or as abnormal coronary blood flow in response to low doses of ACh.43 Using this broad definition of a disturbed vasodilative response, two-thirds of all 1,552 patients with chest pain and non-obstructive CAD had microvascular dysfunction. Both tests of coronary microvascular vasodilatation were normal in 520 patients and abnormal in 268 patients. Divergent findings were seen in 651 patients: an abnormality in response to low doses of ACh was more common than an abnormal reaction to adenosine. Thus, the combination of an abnormality in response to ACh and a normal response to adenosine was seen in 478 patients, whereas the contrary was present in only 173 patients. Hence, endothelial function (vasodilatation) testing using ACh produces many more abnormal results than does standard vasodilatation testing using adenosine. In fact, this form of vasodilatation testing is used in very few centres outside the US today, possibly reflecting the absence of data indicating a better guidance to therapy or a more precise prediction of outcome as compared with adenosine vasodilatation testing. Moreover, the assumption that these two tests measure two different things, namely, predominantly or even exclusively endothelial versus non-endothelial (smooth muscle cell function), may not be correct.48–51
Assessment of Coronary Vasoconstriction or Spasm
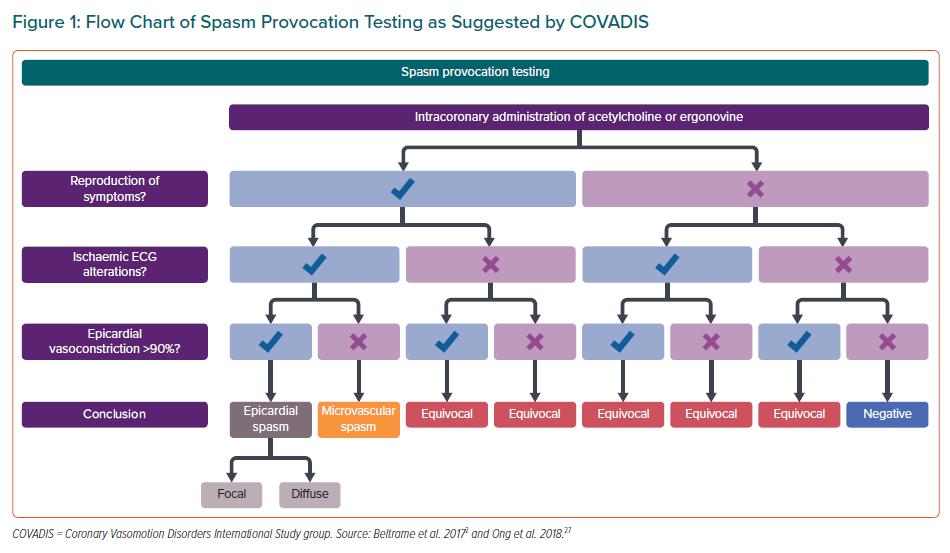
Besides the impaired vasodilatation of the coronary vasculature, coronary vasomotion may also be dysfunctional because of an increased vasoconstrictive potential. This abnormality may not only affect the coronary microvasculature but also the epicardial vessels. Vasoconstriction may occur spontaneously and lead to transient subtotal or even total coronary occlusion (spasm). These spasms frequently occur at rest in patients without obstructive CAD. Fortunately, most of these events are self-limiting, which, however, will hamper the diagnosis of spontaneous events. Thus, in order to reproduce spontaneously occurring events, a so-called provocation test is often required. This test permits assessment of coronary vasomotion as an extension to diagnostic coronary angiography. Different vasoconstriction-provoking agents may be applied. This diagnostic tool may be helpful in identifying patients with coronary vasospasms, which are associated with an increased risk of recurrent angina, repeated coronary angiography and MI.52
For the diagnosis of epicardial coronary spasm, the Japanese Circulation Society (JCS), in their guidelines for diagnosis and treatment of patients with vasospastic angina (VSA), have suggested a definition of coronary artery spasm as a >90% reduction of the epicardial vessel diameter. This may occur as a focal spasm (in one isolated segment of the coronary artery) and/or as a diffuse spasm (occurring in ≥2 adjacent segments, usually in the distal portion of the vessel).53
In contrast to epicardial vasospasms, direct visualisation of microvascular spasm (MVS) during spasm testing is not possible due to the limited spatial resolution of coronary angiography. According to the COVADIS definition, a diagnosis of MVS should be made if ACh causes the known angina symptoms accompanied by ischaemic ECG alterations without any demonstrable epicardial spasm (Figure 1).27 Sometimes, MVS will lead to an abrupt increase in microvascular resistance, causing a dramatic decrease in blood flow velocity.54 However, symptoms may be felt differently by the patient in the atmosphere of a busy catheterisation laboratory, and ECG changes may be ambiguous, such as, for instance, the appearance of negative T waves.
Acetylcholine
ACh is also used for assessing the vasoconstrictive tendency of coronary arteries, taking into account that its binding sites are not only on the endothelium but also on the vascular smooth muscle cells.55 For spasm provocation testing, increasing ACh doses of up to 200 µg are injected into the coronary arteries.53,56 However, protocols differ between institutions, and different injection times and different maximal doses of ACh are in use.56,57 Recently, we summarised how we perform ACh testing in our catheterisation laboratory and how we deal with side-effects such as AF and bradycardia.56,58,59
Undoubtedly, higher doses of ACh provoke coronary vasospasm more frequently than lower doses. In the testing of almost 1,400 patients with stable angina and unobstructed coronary arteries we found pathologic ACh tests in almost 60%. More than half of the abnormal tests were positive for MVS. A pathological test was more common in female patients (70% versus 43%). Female patients were more sensitive to ACh and abnormal tests occurred at lower ACh doses compared with male patients. All of these tests were performed using the slow injection protocol, in which each dose of ACh is given by slow intracoronary injection over 3 minutes.56
Although the criteria for the diagnosis of a pathologic ACh test are commonly accepted, testing may also elicit indeterminate or inconclusive responses.2,27,53 Such responses can be observed in up to one-third of patients.60 This includes reproduction of the usual chest pain without ECG changes or epicardial spasm, the occurrence of epicardial spasm without symptoms, or the observation of ischaemic ST shifts in the ECG without symptoms. All these responses appear to be abnormal but they do not fulfil the criteria for a pathologic test. Some of these patients may have what has been termed ‘the sensitive heart’ by Richard Cannon.61 Hence these indeterminate responses are often grouped together with normal test results.
It obviously makes a difference whether a dose of ACh is given over 3 minutes or 20 seconds. The fast approach is favoured by most Asian centres and there are obvious advantages of following such a protocol.53 Provocation testing can be completed in a much shorter time interval3,62 and the proportion of patients with positive tests seems to be higher. Sueda and Kohno examined 30 patients with ischaemic heart disease and administered up to 200 µg of ACh both over 3 minutes and over 20 seconds.63 They found that more patients had spasms during the 20-second injection than during the 3-minute injection (22 versus 10). Moreover, both ischaemic ECG changes and chest symptoms were significantly more frequently seen with short ACh injection. Interestingly only epicardial spasm was considered in that study. Moreover, the 3-minute protocol was carried out only at the highest tolerable dose previously administered during the 20-second protocol. Patients could receive nitrates if spasms following the 20-second protocol did not resolve spontaneously in 3 minutes. The different outcomes of both test protocols in the same patients invite questions about the sensitivity and specificity of ACh testing in patients with ANOCA. There is no gold standard independent test for comparison. Initially, the test was introduced to find a cause for angina in patients with unobstructed coronary arteries. Thus, the gold standard was the reproduction of the usual angina symptoms that had led to the initial investigation. These angina symptoms were more convincing when associated with ischaemic changes in the ECG with or without epicardial spasm. It is ethically very difficult to perform the test in persons who have no symptoms and no coronary plaques. Such persons would form a cohort of normal people in whom the test should be 100% negative. In contrast, there is also the opinion that the test might be positive in every person if the dose of ACh were high enough. Animal models are difficult because the response to ACh differs widely from species to species.64 We are not aware of animal experiments in which very high doses of ACh were applied to determine whether coronary constriction could be elicited in all animals.
Ergonovine
Besides ACh, current guidelines recommend intracoronary injections of ER as an alternative provocation test.1,53 ER binds to serotonin receptors on the smooth muscle cells and therefore involves mediators other than ACh.65 This is the reason why Sueda et al. recommend using both substances for provocation testing.66 Focal epicardial spasms can be detected more often when ER is used as the provocation agent, whereas diffuse distal spasms occur more often when using ACh.60,66 Also, the proportion of patients diagnosed with coronary spasm may increase by using both agents.66 In the early experience with provocation testing using ER, an IV (and therefore unselective) application was used, which led to a lower frequency of provoked spasms compared with intracoronary application.66 Also, increased complication rates have been described for IV application of ER due to the possibility of prolonged spasm affecting multiple coronary arteries.4 Current guidelines therefore strongly favour intracoronary instead of IV injection of ER.1,53
Lactate Concentration as an Objective Indicator of Microvascular Spasm
Objective detection of the presence of ischaemia during functional testing is important, especially for the diagnosis of MVS. At the moment, one usually relies on whether ischaemic changes can be seen on the ECG because MVS cannot be visualised angiographically. Many patients with MVS report exact reproduction of their usual symptoms and this is associated with horizontal ST segment depression >1 mm. However, despite reproduction of the usual symptoms, some patients show negative T waves and there has been debate on whether this should be interpreted as evidence of MVS. Then there are those whose symptoms are not really the same as in daily life despite horizontal ST-segment depression. Thus, there is a grey zone, and this might affect the reproducibility of test interpretation. Also, the interobserver reproducibility of the diagnosis of MVS has not been reported. An additional and potentially more objective method for proving ischaemia during provocation testing is the measurement of lactate concentration in the coronary sinus, as recommended by the JCS.53
Lactate metabolism by the myocardium encompasses both lactate production and lactate consumption given that lactate is used by the heart as a source of energy. This leads to a net effect of lactate consumption in healthy individuals. During ischaemia, however, the lack of oxygen is associated with an increase in anaerobic glycolysis, resulting in net lactate production.67 Lactate concentration in the coronary sinus will thus increase during ischaemia. The extent of lactate production during MVS can be quantified from paired blood samples taken from the coronary sinus and the aorta at baseline and following the highest tolerable dose of ACh.68,69 A negative lactate extraction ratio (the ratio of the arteriovenous difference in lactate concentration to arterial lactate concentration, negative values indicating myocardial lactate production with coronary sinus concentration exceeding arterial concentration) can be used as an objective measure of the presence of myocardial ischemia.69,70 Therefore, the lactate extraction ratio may also be helpful for quantifying the severity of ischemia: larger amounts of net lactate production, which are associated with more extensive ischaemia, will result in more negative values of the lactate extraction ratio. The use of lactate to diagnose the presence of myocardial ischaemia has thus been recommended by the current guidelines of the JCS for the diagnosis and treatment of patients with VSA.53 Lactate measurements also permit the objective diagnosis of ischaemia due to MVS occurring before the onset of epicardial spasm at higher doses of ACh.69,71 However, lactate measurements require right heart catheterisation and selective catheterisation of the coronary sinus. Moreover, a dedicated point-of-care measurement device needs to be present in the catheterisation laboratory in order to measure lactate immediately following its procurement.
Sequence of Testing: Vasodilatation or Vasoconstriction Testing First?
Although the aforementioned methods for testing coronary vasomotion are increasingly being applied, there is an ongoing debate about the sequence of testing, which focuses on the use of nitroglycerin and the duration of its vasodilative effects on the coronary vasculature.20,31,72 In patients in whom an interventional diagnostic procedure (IDP) is likely to be performed and who are catheterised via the radial artery, the routine use of intra-arterial spasm prophylactic medications such as nitroglycerin and/or calcium channel blockers should be avoided. The smallest catheter size should be used to avoid spasm in the radial and brachial arteries. Focal epicardial spasm may mimic fixed coronary stenosis, and many centres perform diagnostic coronary angiography only after the application of nitrates, although this practice differs between centres.73,74 We recommend avoiding nitrates before diagnostic coronary angiography if performing an IDP.
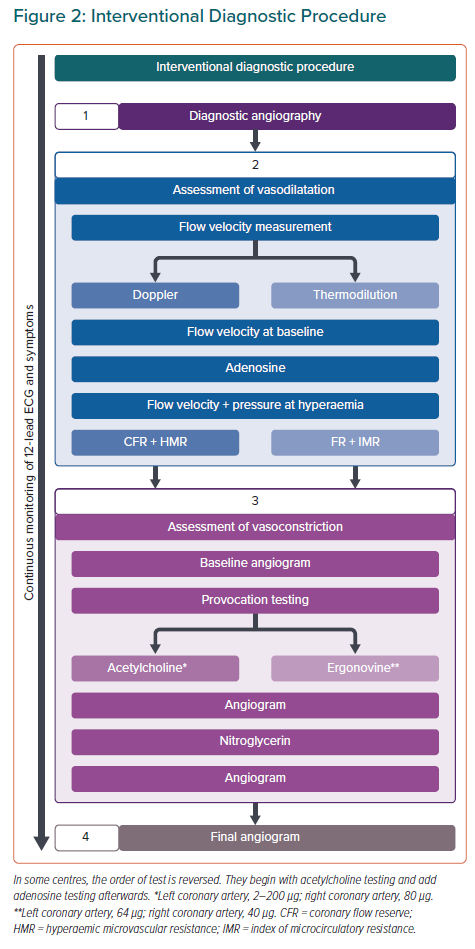
The advocates of starting the IDP with vasodilatation testing using adenosine argue that some patients require wire-based measurements (fractional flow reserve [FFR], instantaneous wave-free ratio etc.) to exclude haemodynamically significant epicardial stenoses in the presence of coronary plaques. Thus, a guidewire needs to be placed first and adenosine will be given if FFR is measured. Some centres give intracoronary nitroglycerin (chemical name, glyceryl trinitrate) routinely before the use of adenosine to ascertain maximal vasodilatation. However, this is not a necessary part of the FFR or the CFR procedures.75 Hence, it appears reasonable to perform the vasodilatation testing part (determination of CFR and IMR or HMR) first, keeping in mind that the use of nitroglycerin or calcium channel blockers should be avoided if possible.
However, there are no data on how the prior application of nitroglycerin will affect ACh testing. The two most interesting questions are whether the proportion of pathologic ACh tests will decrease if nitroglycerin has been given before the test, and how long the recommended time delay should be to ensure that prior nitroglycerin does not affect the ACh test results. There is, however, much speculation. The authors of the CorMicA trial argue that at 10 minutes after the application of nitroglycerin, only 3% of the substance will still be present and active due to the short half-life of around 2 minutes of the substance.72 Consequently, they find it unlikely that at 10 minutes after the application of the nitrate the remaining 3% of the compound might lead to false-negative results of ACh testing. In contrast, Morton Kern remarks: “NTG dilates and fixes the diameter of the epicardial vessel for 10–15 minutes after 100–200 mcg i.c.”76 If one suspects a longer duration of the action of nitroglycerin, this would indeed argue against performing the ACh test first. This is because nitroglycerin should be routinely given at the end of the ACh test.53 The reason for giving nitroglycerin at the end of the procedure is that the extent of coronary vasoconstriction with ACh has been quantified, as compared with the relaxed state of the artery following nitroglycerin. In addition, residual spasm may be reversed. Indeed, by injecting nitroglycerin it is more likely that the criterion of a >90% narrowing can be fulfilled and a diagnosis of epicardial spasm made, than if the reference diameter is the arterial diameter at baseline.74 Despite their assumption that nitroglycerin will have little effect 10 minutes after its application, Ford and Berry are reluctant to perform the ACh test first. This is because they feel that ACh-induced spasm might lead to false measurements of resting flow and hence CFR.72 They suspect that the provocation of spasm and subsequent ischaemia (which may be prolonged following provocation of MVS) could lead to reflex hyperaemia, which might result in falsely high values of resting blood flow. Thus, CFR would be falsely low. If, in contrast, MVS persists, resting blood flow would be falsely low, resulting in a potentially falsely high CFR. Using Doppler-based flow velocity measurements both before and after provocation testing may solve this dilemma by identifying the right time to proceed, namely after normalisation of coronary blood flow velocity.
The opposing view assumed a larger half-life of nitrates in blood77 of up to 6 minutes, leading to >25% remaining in blood 10 minutes after its application. In addition, the vasodilating effects of nitrates on epicardial coronary arteries may last even longer than those on the coronary microvasculature, as demonstrated in a canine model (45 versus 7 minutes).78 This argument (falsely) assumes that intracoronary nitrates are required for the adenosine measurements. Therefore, the best approach for measurements not affected by residual nitroglycerin effects will be to use the smallest possible catheters for coronary angiography and vasomotion testing. This would reduce the prevalence of radial spasm and omit the need for a radial cocktail unless radial spasm occurs.79 Performing adenosine testing first without nitroglycerin is the logical choice. ACh testing can be done afterwards without undue influence of any residual vasoactive substances (Figure 2). This sequence also avoids discussions about persisting MVS. It also has the potential advantage that the delay time between any application of a vasodilating substance in the case of a radial spasm and the ACh test would be maximised.
Endotypes
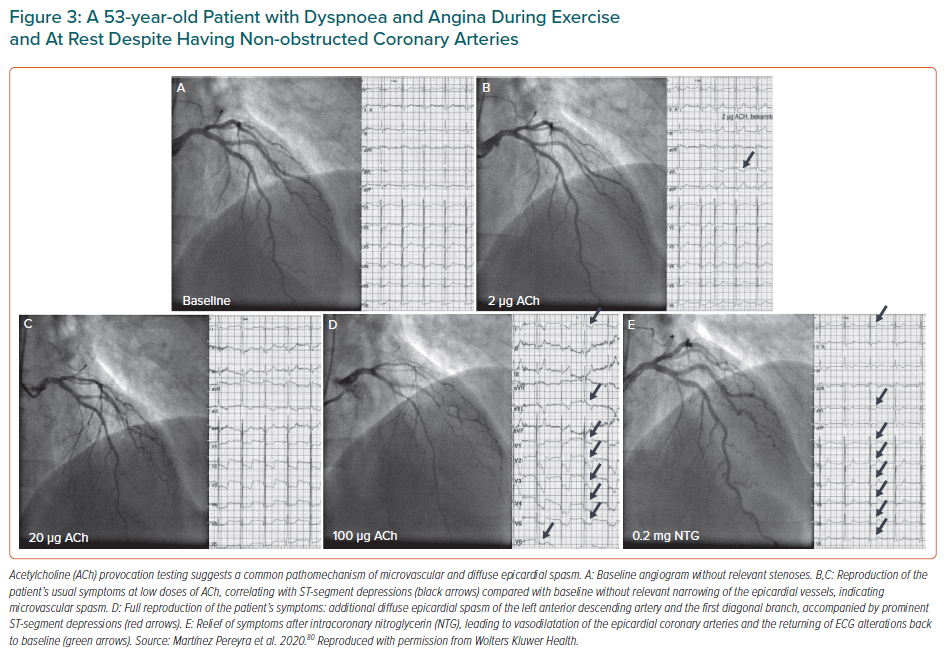
If the aforementioned assessments of vasodilatation and vasoconstriction are done immediately following clinically indicated coronary angiography, no additional invasive procedure is required for the diagnosis of coronary vasomotion disorders. In the CorMicA trial, Ford et al. used a pathway of testing termed the IDP.38 After diagnostic coronary angiography via the radial artery for exclusion of obstructive CAD, assessment of vasodilatation was performed first, followed by ACh endothelial testing and spasm provocation testing. Measurement of maximal vasodilatation was achieved by IV infusion of 140 µg/kg/min adenosine. Depending on the results of each assessment, the following endotypes have been classified for patients without obstructive CAD: endotype 1, microvascular angina (MVA; impaired vasodilatation and/or MVS); endotype 2, VSA (epicardial spasm); endotype 3, mixed MVA and VSA (epicardial spasm + impaired vasodilatation); and endotype 4, non-cardiac chest pain. This classification distinguishes between MVS (endotype 1) and epicardial spasm (endotypes 2 and 3), following different therapeutic recommendations. However, sometimes patients may exhibit MVS at lower doses of ACh, followed by (diffuse) epicardial spasm at a higher dose.69,80 Such observations suggest a common pathomechanism of diffuse distal epicardial spasm and MVS (Figure 3). One might therefore adjust the definition of endotypes according to the type of vasomotion that has been found to be impaired when assessing vasodilatation and vasoconstriction: both tests (vasodilatation and vasoconstriction) can be abnormal, one can be normal and the other abnormal, or both can be normal. This new classification has been applied by current ESC guidelines, which recommend the same therapy for both epicardial spasm and MVS. For impairments in vasodilatation, the therapy differs. In the case of both mechanisms being impaired, the medical therapy shall focus on treating the (suspected) dominant mechanism.1
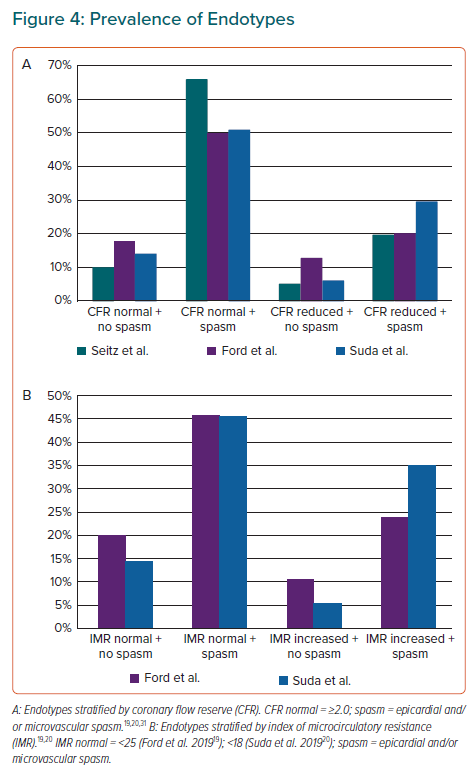
Further variations between centres relate to the type of flow velocity measurement used (Doppler versus thermodilution), the ACh injection speed, the incremental dosages of ACh used or the standardised usage of a pacemaker during provocation testing.19,20,31 As a consequence, the prevalence of the four endotypes might differ between centres due to the different protocols and threshold values applied. Furthermore, some groups prefer other definitions of endotypes that incorporate information about endothelial function, the type of spasm encountered (focal versus diffuse versus microvascular) and so on.31,43 This not only makes it hard to find a common language for discussion but it also makes it difficult to compare results between centres. The definitions of subgroups, in particular, are inconsistent. For instance, Ford et al. use the term ‘microvascular angina’ to describe a group of patients in whom the vasodilative potential is impaired (reduced CFR and/or increased IMR), or in whom the vasoconstrictive potential of the microvasculature is increased (MVS), or in whom both mechanisms are impaired in parallel.19 Even though this classification makes sense when explaining the microvascular origin of the disease, it does not distinguish between the two pathomechanisms for microvascular dysfunction (i.e. impaired vasodilatation versus enhanced vasoconstriction) and thus cannot be used to select the optimal treatment.
Suda et al., in contrast, did not use these classifications, however, they use the term ‘negative test’ in two different ways.20 First, the term ‘negative’ encompasses a group of patients without epicardial spasm and with normal vasodilative properties, even though this subgroup contains patients with and without MVS. In another part of the study, the negative group contained patients without any type of spasm. This may illustrate how terminology makes the comparison of results between different groups very complex. Ideally, the term chosen should be self-explanatory and used in a unique, well-defined sense, thereby reducing the possibility of misunderstandings.
If endotypes are defined in the straightforward way as described above (vasodilatation testing and vasoconstriction testing both abnormal, either of them abnormal or both normal) and this is applied to the three studies providing such information, then isolated coronary spasm without accompanying impairment of vasodilatation (Figure 4) is the most prevalent endotype.19,20,31 Interestingly, one encounters only a few patients (around 10%; Figure 4) with an isolated impairment of vasodilatation and without accompanying spasm. These patients have what has been called ‘microvascular angina’, given that this term previously did not include angina secondary to MVS. However, as judged from the available literature, this subgroup of patients would be expected to be the most prevalent one. Finally, the recent expert opinion paper also struggled with the definition of microvascular angina.81 Although in Figure 4 of this expert opinion paper, this group is defined as having an abnormal adenosine test (vasodilatation test) but a normal ACh test, in table 2 of the same publication the patients with microvascular angina are characterised as having an abnormal adenosine test and/or an abnormal ACh test (i.e. they may be positive for MVS).81 Hence, it is mandatory that agreement is reached on the definition of endotypes. Only then will we have the basis for the multicentre therapeutic and prognostic studies that are urgently needed.
Conclusion
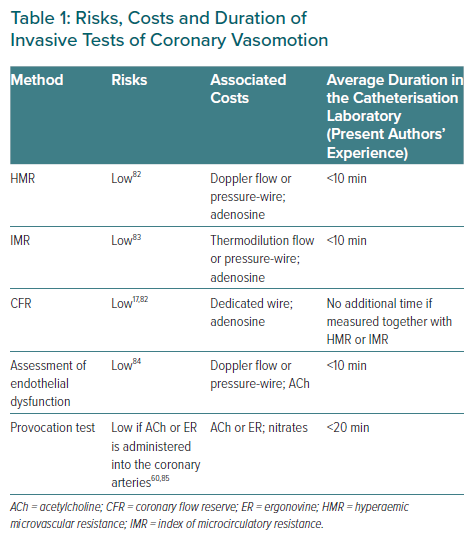
The methods reviewed in this article can be easily and safely applied by the invasive cardiologist in order to diagnose coronary vasomotion disorders. However, the number of centres routinely implementing coronary vasomotion testing is low, even though neither additional risks nor costs are associated with these tests (Table 1). This is especially true if a wire has already been placed in the coronary artery for another reason, for instance to measure FFR in the presence of coronary plaque. In addition, in Germany the IDP is also reimbursed if performed in smooth coronary arteries in a patient with suspected functional coronary abnormalities, due to separate coding information. Thus, there is no convincing argument for not performing such testing, especially in view of the advantages of clearly identifying the cause of the patient’s symptoms.38
The hesitation prevailing in many catheterisation laboratories with regard to adopting IDPs may be related to the fact that the complexity of coronary function abnormalities is still not fully understood and will require further research and development to improve diagnosis and treatment. Nevertheless, extending intracoronary testing such as the measurement of FFR or of resting flow indices such as instantaneous wave-free ratio or resting full-cycle ratio in the presence of coronary stenoses to the field of coronary function is rather simple. Such testing will expand the scope of the invasive cardiologist and will open a new window onto the fascinating biology and pathophysiology of coronary arteries.