There has been a momentous rise in the number of female athletes competing in the Olympics and the highest ranks of sports, yet women remain understudied. It is vital to recognise the impact of intensive exercise on the female athlete’s heart. It is well known that regular intensive exercise is associated with several electrical and cardiac structural manifestations that lead to a larger stroke volume to generate and deliver an increased cardiac output for prolonged periods.1,2
The chromosomal variation between men and women results in several differences, including physiological, psychological and biochemical variations, that will influence their cardiovascular adaptation to exercise. Women are generally smaller, with a lower lean body mass, higher circulating oestrogens and lower androgen levels to produce the power and momentum for sports.3
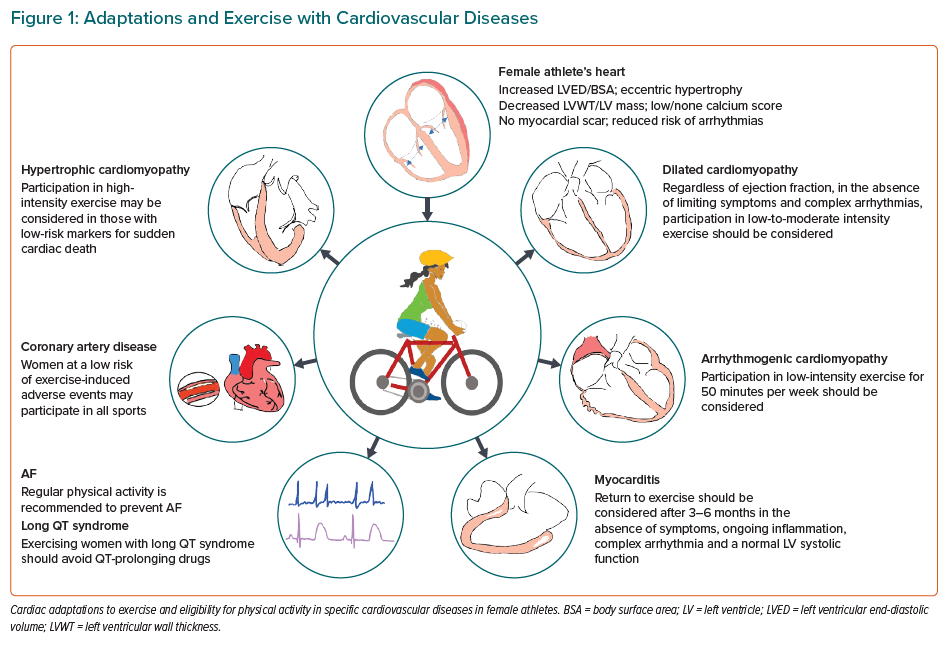
This review will focus on the management of cardiovascular disorders in female athletes.
The Female Athlete’s Heart
Although female athletes develop similar qualitative cardiac structural changes to their male athletic counterparts, these appear to be on a smaller scale.
A principal study performed in female athletes by Pelliccia et al. compared predominantly 600 white women with 66 sedentary controls.4 The women in this study were relatively young, with a mean age of 21 years, an exercise history of more than 9 years and participated in 27 sporting disciplines, including swimming, roller skating, track events and gymnastics. The research found that compared with controls, female athletes had a 6% thicker left ventricle (LV) wall and a 14% greater LV cavity size (LV end-diastolic, 49 ± 4 mm versus 46 ± 3 mm; p<0.001). In absolute terms, 8% of women had an enlarged LV cavity (>54 mm) and 1% had a LV cavity of >60 mm that could be comparable to a dilated cardiomyopathy. In comparison to male athletes, women had smaller absolute LV cavity dimensions, with 48% of men achieving a LV end-diastolic dimension of >54 mm. Irrespective of sex, determinants of cardiac size include age, ethnicity and sporting discipline with the largest dimensions identified in those who engage in endurance sport.5
Right ventricle (RV) dilatation occurs in response to increased cardiac preload and is often identified in endurance athletes. RV size parallels changes found in the LV and physiologically these should be symmetrical without differences in the RV:LV ratio.6–8 Female athletes generally have smaller absolute RV dimensions than male athletes but, when indexed to body surface area, female athletes have larger RV dimensions.8
Female athletes generally do not achieve a LV wall thickness >11 mm, but are able to develop a large LV cavity size.9 Recently, female athletes’ hearts were evaluated with LV geometry, which takes into account the LV mass and relative LV wall thickness (ratio of LV wall thickness to cavity size).10 Normal geometry is defined as a normal LV mass and relative wall thickness <0.42; concentric remodelling is defined as a normal LV mass and increased relative wall thickness (>0.42); concentric LV hypertrophy is defined as an increased LV mass and a relative wall thickness; and eccentric LV hypertrophy is defined as an increased LV mass and normal relative wall thickness.11
Finocchiaro et al.10 studied LV geometry in 1,083 young adult athletes including 443 women competing in several sports. They identified that 22% of female athletes showed abnormal geometry with a higher prevalence of eccentric LV hypertrophy in those engaging in endurance sports. In contrast, male athletes are more likely to develop concentric hypertrophy (9% versus 3%; p<0.001). None of the women exhibited a relative LV wall thickness of >0.48, which suggests that female hearts adapt by increasing their LV cavity size. This parameter may be used to differentiate abnormal LV hypertrophy from physiological changes rather than absolute LV wall thickness measurements.
Furthermore, Rawlins et al. assessed how ethnic differences affect cardiac adaptations in 400 female athletes.12 The authors demonstrated a greater LV wall thickness (9.2 ± 1.2 mm versus 8.6 ± 1.2 mm; p<0.001) and LV mass (187 ± 42 g versus 172 ± 42 g; p<0.008) in black compared with white female athletes. Eight black female athletes (3%) exhibited a LV wall thickness >11 mm (12–13 mm), but none of the white female athletes did. All female athletes demonstrated normal indices of systolic and diastolic function. Therefore, a LV wall thickness of >13mm in a black female athlete is rare and warrants further evaluation.
Left atrial enlargement occurs in correlation with LV dilatation secondary to high preload imposed on all four chambers with intensive endurance exercise.13 Female athletes have a greater absolute and indexed atrial volumes compared with controls. An indexed left atrial volume of 22–33 mm/m2 has been suggested in women.14
The Female Athlete’s ECG
The athlete’s ECG reflects changes in vagal tone and cardiac dimensions. Women generally show smaller quantitative changes with a lower prevalence for voltage criteria for biventricular hypertrophy, and shorter PR interval and QRS duration.10
Women have different patterns of T-wave inversion from male athletes. Minor anterior precordial T-wave inversion is more common in women, particularly when limited to leads V1–V3, and has not been associated with underlying structural heart disease.10,15,16 The current international recommendations on ECG interpretations in athletes suggest T-wave inversion in leads V1–V2 in women does not warrant further evaluation in the absence of symptoms or a family history of cardiomyopathy.17 In contrast, T-wave inversion in the lateral and inferior leads in female athletes is rare and should raise concerns of pathological LV hypertrophy. Women in general have a longer corrected QT interval than men. Based on international recommendations, a QTc >480 ms warrant further evaluation for long QT syndrome in female athletes.17
Cardiovascular Diseases in Female Athletes
Cardiomyopathies
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is defined as LV or biventricular systolic impairment with or without cavity enlargement that is not explained by coronary disease or abnormal loading conditions.18 It has several causes including genetic predisposition, myocarditis, tachycardia-induced or ventricular premature beat-induced cardiomyopathy, drugs, toxins, peripartum cardiomyopathy and, occasionally, the cumulative effects of more than one aetiology.18
The clinical spectrum of DCM may vary from a phenotype with the absence of symptoms, isolated LV dilatation and normal or low-normal systolic function to overt disease with significant systolic dysfunction.
Some athletes engaging in endurance sport, such as long-distance running, swimming and rowing, may exhibit large cardiac dimensions that overlap with those observed in patients with DCM. The differentiation between cardiac pathology and physiological adaptation is fundamental to prevent a wrong diagnosis and fatal consequences. A dilated left ventricle of >60 mm consistent with a DCM is identified in only 1% of female athletes.4 These women should be evaluated thoroughly with a detailed clinical history, including any relevant family history of cardiomyopathy. They should be investigated with cardiovascular MRI to exclude myocardial fibrosis, prolonged ECG analysis for significant arrhythmias and exercise echocardiography for their haemodynamic response to exercise, to assess for presence of exercise-induced symptoms or arrhythmias and to evaluate for a contractile reserve of >11% to exclude cardiac pathology.
In general, symptomatic women with DCM should abstain from most competitive and leisure sports associated with moderate or high-intensity exercise.19 A select group of female athletes with DCM who have mildly impaired LV ejection fraction of 45–50% without high-risk markers or arrhythmias may participate in most competitive sport.19 Annual follow-up is recommended for females with DCM who exercise regularly (Figure 1).
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is diagnosed in the presence of an unexplained increase in LV thickness to ≥15 mm in end-diastole in any myocardial segment.20 HCM may also be considered in women with a LV wall thickness of ≥13 mm in the presence of a positive genetic test or family history of HCM.20
Women and men should be equally affected given the autosomal dominance of this disease; however, this is not reflected in the literature. Although there may be a lack of awareness of cardiovascular disease (CVD) in women, it is possible that hormonal influences and genetics play a role. Oestrogens have a protective role as women usually have an older age of onset, a lower LV mass and a secondary hypertrophic response.
Female athletes rarely succumb to sudden cardiac death (SCD) from HCM. In the US National Registry, women accounted for only 3% of 302 individuals who died of HCM.21 This observation is relevant to the prescription of competitive sports and intensive exercise in this cohort. White female athletes rarely exceed a LV wall thickness >11 mm and around 3% of black female athletes will have a LV wall thickness between 12 mm and 13 mm.9,12 Therefore, a LV wall thickness of 12 mm in a female athlete may fall into a grey zone depending on body size, sporting discipline and ethnicity, and may potentially raise concern for pathology, such as HCM.
A systematic approach is required when assessing women with HCM or the suspicion of pathological HCM who request exercise advice. The baseline evaluation should include a comprehensive personal and family history, assessment of the severity of the HCM phenotype and the presence of conventional risk factors for SCD. Evidence has been evolving over the past two decades that exercise is beneficial with low rates of adverse events in adults with mild phenotypes of HCM.22 A more liberal approach to competitive sports participation and intensive exercise has been adopted in individuals with morphologically mild HCM and a low 5-year HCM risk of sudden cardiac death score of <4% (Figure 1).23
Arrhythmogenic Cardiomyopathy
Arrhythmogenic cardiomyopathy (AC) is defined by the presence of fibro-fatty replacement and myocardial fibrosis of both or individual ventricles and life-threatening ventricular arrhythmias. It is also recognised that intensive exercise and endurance sport in individuals with AC accelerates the phenotype.24
AC is an autosomal dominant disease with an equal number of men and women genetically affected. However, several large studies have shown women are less phenotypically affected than the men, possibly owing to different levels of participation in competitive sport and/or endocrine influences.25–29 Studies in mice suggest androgen levels contribute to the advancement of the disease process. The androgen receptors in the heart are upregulated with exercise, which may be a contributory factor for the disease progression, and female athletes usually have a lower number of androgen receptors in their hearts than men.30
One-third of athletes will exceed the cut-off values in RV dimensions for the minor criteria defining AC. Consequently, in female athletes with a disproportionate ratio of right to ventricular end-diastolic volume (RVEDV:LVEDV) of >1.3 should undergo a detailed evaluation in the presence of symptoms and/or relevant family history of cardiomyopathy.6
It is also recognised that intensive exercise and endurance sport in individuals with AC exacerbates the phenotype. A cautious approach is therefore recommended in people with AC, including those who are genotype positive but phenotype negative. Participation in low to moderate intensity recreational exercise may be considered for individuals without a history of cardiac arrest, ventricular arrhythmias or unexplained syncope with minimal structural cardiac abnormalities or <500 premature ventricular complexes in 24 hours (Figure 1).19
Myocarditis
Myocarditis is a non-ischaemic inflammatory myocardial disease that may cause cardiac dysfunction and complex arrhythmias. In the developed world, viral myocarditis is the most common aetiology.31,32 The precise incidence and prevalence of myocarditis is unknown. It accounts for 5–15% of SCD in young athletic individuals.33–37 Daily exercise in mice models infected with the coxsackie virus was associated with increased predisposition to fulminant myocarditis and sudden death.38
Testosterone levels are associated with increase fibrosis and heart failure.39 SCD during sports from myocarditis appears to be more prevalent in men than women, which may be linked to the testosterone-related inflammatory response.33,34
The clinical presentation of myocarditis is heterogenous and patients may initially present with coryzal symptoms. Athletic individuals may exhibit non-specific symptoms of general malaise, fatigue or diarrhoea.40 At the other extreme, individuals can present with fatal arrhythmias and cardiogenic shock. Almost 50% of people with myocarditis will demonstrate full resolution of LV dysfunction within 30 days but 12–25% will progress to fulminant heart failure that is an adverse prognostic marker in the long term.33,41
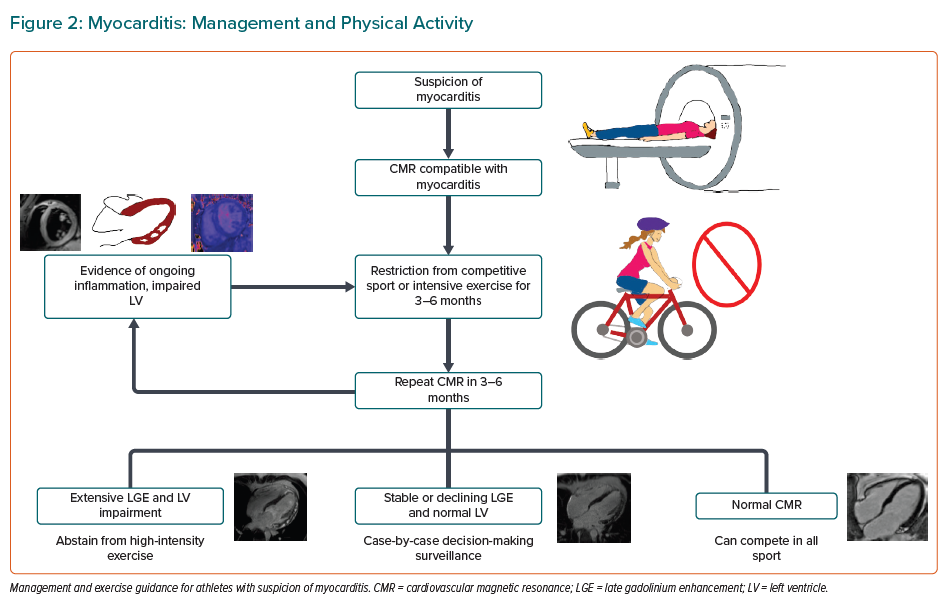
Currently, there is no single clinical or imaging marker to confirm the diagnosis of myocarditis with certainty. In the current era, cardiovascular MRI is the most useful diagnostic and prognostic test with an excellent sensitivity and specificity for detecting myocardial hyperaemia, inflammation, oedema and/or focal fibrosis.42 The extent (>10% increase) and distribution (anteroseptal) of myocardial fibrosis are important predictors of cardiovascular events that should be assessed during clinical follow-up.43–45
Intensive exercise should be avoided in individuals with myocarditis. Women with active myocarditis should not engage in sport for 3–6 months and should return to intensive exercise only when LV function is normal and no complex ventricular arrhythmias are seen on an exercise stress test (Figures 1 and 2).19
Coronavirus Disease 2019
Athletic individuals infected with severe acute respiratory syndrome coronavirus 2 may be at risk of a subclinical myocarditis and may remain asymptomatic or develop mild symptoms.46 Women appear to be less severely affected by coronavirus disease 2019 (COVID-19) than men and have a lower prevalence of cardiovascular injury.47,48 It is likely that levels of ACE2 expression and sex hormones regulate these changes and control systemic inflammation.49–51
Athletes who test positive for COVID-19 and exhibit isolated cardiac symptoms are required to refrain from high-intensity physical activity and sport for at least 7–14 days since they are symptom free.52,53 Following resolution of symptoms, athletes with a negative COVID-19 test should undertake cardiac testing including a high-sensitive troponin test, an ECG and echocardiogram to exclude cardiac involvement.52,53 Abnormalities specifically relating to myocarditis warrant a 3–6 month sports restriction.
More recently, rare cases of myocardial and pericardial inflammation following COVID-19 vaccination have been reported.54,55 The Centers for Disease Control and Prevention reported an incidence of 4.8 cases per 1 million between COVID-19 mRNA vaccines and myocarditis, particularly in young men within a few days of the second vaccination.56 Pericarditis tended to affect older patients after the first or second dose. Women also experience post-vaccination cardiac inflammation but to a lesser degree.54
The recommendations for the management of exercising after COVID-19 are likely to evolve over time as long-term data are acquired in this highly infectious virus.
Hypertension
Hypertension is common in the global population, affecting around one-third of people. Exercise is an effective treatment strategy for hypertension but conversely, exercise-induced hypertension may equally increase the risk of cardiovascular events. The precise prevalence of hypertension in female athletes is unknown as definitions in the literature vary but it is likely to be similar to that identified in the general population.57
There is also a lack of consensus in the definition and threshold for abnormalities in exercise-induced hypertension, which carries an elevated cardiovascular risk as it is linked with subclinical hypertension.58,59 Determining factors for hypertension include older age, drugs (i.e. anabolic steroids and nonsteroidal anti-inflammatory drugs), sporting discipline and the intensity of sport.
Few studies in the literature have evaluated sex differences in hypertension.60–63 Female athletes appear to have a lower prevalence of hypertension and lower systolic and diastolic values compared to male athletes in both low and high bodyweight classes.60,61,63 There are no significant differences between various sports although higher blood pressure (BP) readings have been detected in endurance (including swimming, triathlons and pentathlons) and artistic sports.60
Only one study has reported higher BP values in female than male athletes (13% versus 7%) but this difference was likely due to a wide variation in age.62 The study included athletes aged 13–77 years, but men were predominantly younger than 35 years. Of the hypertensive women, 92% were aged >35 years, while 73% of the hypertensive men were >35 years old. Out-of-office BP recordings should be performed in all athletes with BP >140/80 mmHg on physical examination as there is a high prevalence of white-coat syndrome. Hypertensive individuals should participate in at least 30 mins of moderate-intensive dynamic aerobic exercise 5–7 days per week to reduce systolic and diastolic BP of 7 and 5 mmHg, respectively.
Ischaemic Heart Disease
Atherosclerotic coronary artery disease (CAD) is a potentially fatal condition affecting predominantly mature athletes. It is more common in men with a 9:1 ratio for SCD among competitive athletes and is even higher among recreational athletes with prevalence ratio of 20:1.64 Although hormonal influences cannot be disregarded, the likely explanation for this sex difference is the engagement in lower-intensity sports and participation rates in sports among women.65
The majority of studies evaluating CAD have been conducted in male runners. There are few data in female cohorts, demonstrating a low prevalence of CAD.65–67 A study on 152 master athletes, 30% of them women, found no differences in the plaque prevalence and composition compared with sedentary women.65 Similar results had been reported in the same year in a smaller study on 26 female marathon runners; athletes had coronary artery calcium (CAC) counts and calcified plaque volumes comparable to sedentary individuals.66 Another large study on 9,501 women failed to demonstrate an association of high-intensity exercise and high coronary artery calcification scores.67
In athletes with established CAD, there is a potentially increased risk of MI during vigorous physical activity. Therefore, the management of such individuals should focus on risk stratification for recurring events, arrhythmias and inducible ischaemia. Additional evaluation for symptoms of ischaemia, LV ejection fraction <50% and reduced exercise capacity is also warranted. Athletes with any of these high-risk features should be advised to confine themselves to low- and moderate-intensity exercise once symptoms have settled or following a period of 12 months after MI or coronary revascularisation (Figure 1).
Spontaneous Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD), although rare, is a frequent cause of acute coronary syndrome in low-cardiovascular risk, middle-aged women.68,69 SCAD is defined as non-iatrogenic separation of the coronary arterial wall with or without intimal tear by intramural haemorrhage and can be triggered by physical, emotional and hormonal stressors.70 Spontaneous healing is recommended as the first-line therapy.
Data on the risk of exercise triggering coronary dissection are rare so a cautious approach to exercise is generally recommended. Individual assessment for symptoms and inducible myocardial ischaemia is advised before recommencing leisure sport or competitive exercise.19
Mitral Valve Prolapse
Mitral valve prolapse (MVP) is characterised by fibromyxomatous changes of the mitral valve tissue with a >2 mm extension into the left atrial cavity. MVP is more prevalent in women and the most common structural post-mortem cardiac abnormality identified.71–72
Features of increased arrhythmic risk include: female sex; personal history of syncope; family history of SCD or MVP; T-wave inversion, ST-segment elevation, long QTc on ECG; bi-leaflet prolapse; the Pickelhaube sign (high velocity systolic signal with tissue Doppler imaging S-wave >16 cm/s, resembling the pickelhaube spiked helmet); mitral annulus disjunction; complex ventricular arrhythmias on ECG monitoring or exercising testing; and presence of fibrosis on CMR.73–74
The effect of high-intensity exercise on the risk of SCD remains uncertain. In an 8 ± 2 years’ follow-up study on 215 athletes with MVP, no cases of SCD were registered.75 Other adverse events occurred at a rate of 0.5% per annum, mainly in older athletes, with none occurring in athletes with isolated MVP or mild mitral regurgitation.75
Asymptomatic female athletes with MVP and mild or moderate mitral regurgitation can participate in all competitive and recreational sports in the absence of the aforementioned risk markers. In the presence of symptoms or high-risk features, the general advice is to restrict to low-intensity aerobic exercise.22
Arrhythmias
Sex differences in electrophysiological changes are well known. Women have greater sinoatrial node automaticity, atrioventricular node function, infra-Hisian conduction and longer ventricular action potential duration, which may influence the prevalence of arrhythmias in female athletes. However, the vast majority of studies have focused mainly on male athletic populations and sub-data analyses are limited as they are largely underpowered.
Female athletes appear to be at lower risk of bradyarrhythmias and accessory pathways than male athletes, including a higher degree of atrioventricular block.76–78 In contrast, atrioventricular nodal re-entry tachycardias appear to be more prevalent in women.19,76,78
AF
AF is the most common arrhythmia in the general population and is associated with a high risk of heart failure, stroke and mortality. Several studies have revealed a J-shaped relationship between exercise, the risk of developing AF and endurance exercise. The risk of AF is generally reduced at low to moderate intensity exercise (Figure 1).79–81 However, regular vigorous exercise may result in AF in some individuals.78,82–86 Mature endurance athletes generally have a fivefold increased risk of AF.87 However, it remains controversial whether sex-specific differences exist in relation to the risk of AF and long-term endurance exercise.88
The vast majority of data focus on male athletes and a number of studies have failed to prove any increased risk of AF in female athletes.89–91 A meta-analysis that included 103,298 mature female athletes (aged 54–63 years) demonstrated an 8.7% lower risk of AF with moderate-intensity exercise with a further reduction to 28% in women exercising at a high intensity.91 However, female endurance athletes who engaged in very high intensity exercise were underrepresented in this analysis.
In contrast the Norwegian Tromsø Study followed 20,484 subjects, half of them women, for an average of 20 years.88 Healthcare records were intermittently reviewed prospectively to identify cases of AF. Intense endurance training was associated with a twofold increased risk of AF in women aged >40 years. However, moderate-intensity exercise was associated with a significantly lower risk of AF over the 20-year follow-up period.
A more recent study of 402,406 individuals (52.5% women) aged 40–69 years with 2.8 million person-years of follow-up reported a number of points pertaining to women.79 Physical activity of >500 metabolic equivalent-minutes per week (MET-min/wk) was associated with reduced risk of AF, which was more pronounced in women than men. In women, incident AF declined with physical activity of up to 5,000 MET-min/wk compared with men who demonstrated a moderate decline.
Female athletes who develop AF without a substantial cumulative effect of exercise should be evaluated for typical causes of AF such as thyrotoxicosis, valvular heart disease, alcohol abuse, hypertension and ischaemic heart disease. AF ablation is recommended in exercising individuals with recurrent symptomatic AF and/or those who do not want drug therapy because of its impact on athletic performance.19 The athlete should also be counselled on the effect of long-lasting intensive exercise on AF recurrence. Direct bodily contact sports are not recommended in athletes with AF who are anticoagulated.19
Long QT Syndrome
Long QT syndrome (LQTS) is a genetic cardiac disorder characterised by a prolonged QT interval on the ECG and is associated with the increased risk of life-threatening arrhythmias, particularly stress-induced torsades de pointes.92
Healthy women have longer QT intervals than men, which appears in the post-pubertal phase, owing to a longer ventricular action potential.
Recently published clinical observations demonstrated that exercise could induce repolarisation prolongation and suggest LQTS.93 Differences in cardiac repolarisation in women and men have also been reported in LQTS.94 Therefore, the upper limits of normal corrected QT are higher in female athletes than in men (480 ms versus 470 ms in female and male athletes, respectively).77
Women are more likely to be affected by LQTS, despite the equal sex distribution of the disease genotype. They are also at a higher risk of arrhythmias in response to QT-prolonging drugs and electrolyte imbalances than men. Female sex also appears to be an independent risk marker for cardiac events in LQTS.95
Although the data are scarce, given the sex differences, these finding supports the theory that sex hormones most likely regulate cardiac repolarisation.
In the setting of competitive sport, establishing arrhythmic risk can be difficult, particularly in individuals who are genotype positive and phenotype negative. Some studies have reported a lower incidence of arrhythmic events in affected individuals competing in sports but these studies were limited by younger age, participation in low-intensity sports inclusion and their retrospective nature.96–98 In the general adult population, the major risk predictors include a history of cardiac events, QTc values, being a paediatric male or adult female individual as well as the pathogenic mutation in the QT genes.98
It is recommended that all exercising individuals with LQTS with symptoms or prolonged QTc are treated with β-blocker therapy. Individuals should also be advised to avoid QT-prolonging drugs and electrolyte imbalance. Ventricular arrhythmias in LQTS are adrenergically driven; therefore, high-intensity exercise is prohibited even while on beta-blockers in people with a QTc >500 ms or genetically confirmed LQTS with a QTc³ of 480 ms in women. In individuals who are genotype positive and phenotype negative, the European recommendations advise on shared decision making with the athlete in question (Figure 1).19
Veteran Female Athletes
The number of veteran athletes aged 40 years and above engaging in endurance sports including triathlon and marathons has continued to increase with time. There has been a surge in the number of recreational female athletes in this age category. In parallel, there is emergent evidence that chronic exposure to large amounts of endurance exercise may predispose athletes to AF, myocardial fibrosis and increased CAC.65,99–102
Consequently, in some individuals, life-long exercise may be detrimental to an otherwise normal cardiac structure and function. Unfortunately, enrolment of women has been slow in studies evaluating the effects of long-term exercise on the heart to further our understanding of sex differences on cardiac maladaptation. Arrhythmias, myocardial fibrosis, high CAC and atherosclerotic plaque burden appear to be less prevalent in women suggesting oestrogen may play a role in preserving cardiac health.65
A large meta-analysis including 149,000 women demonstrated that long-term vigorous exercise was associated with a 28% reduced risk of AF in female athletes. 79,91
Studies investigating myocardial fibrosis have predominantly focussed on male athletes. In a study, otherwise healthy long-term triathletes of whom one-third were female were investigated with cardiovascular MRI.103 None of the female athletes had myocardial fibrosis compared to 17% of male athletes correlating with a higher peak exercise BP and the number of hours of training. Female athletes generally have a lower peak exercise BP that may account for the difference.
A study by Merghani et al.104 evaluated 170 veteran athletes aged 54 ± 8.5 years, of whom 29% were female, and 132 controls of a similar age, sex and low-risk coronary score for coronary disease. There was a higher prevalence of AF, coronary plaques, myocardial fibrosis and ventricular tachycardia limited to male athletes in comparison with healthy controls. None of the female veteran athletes exhibited adverse cardiac remodelling. Female athletes had a similar burden of coronary disease as sedentary women. The absence of coronary disease in female veteran athletes suggests their genetic and hormonal make-up may hold back adverse arrhythmogenic remodelling.
Sudden Cardiac Death
There is a significantly lower prevalence of exercise-related SCD in women with a ratio of 3:1–10:1, and this also holds true for older recreational athletes, where the men are 20-fold more likely to die of SCD than female athletes.19 There also appear to be sex differences relating to diseases that predispose people to SCD. Female athletes rarely succumb to HCM according to the US National Registry, which is probably due to fewer ventricular arrhythmic events from a reduced volume and intensity of exercise in women.21 However, hormonal and metabolic factors may also play a protective role in reducing the arrhythmic risk during intensive exercise.
In contrast, a different situation exists in women with MVP. In the Italian pathology registry of 650 SCDs, 7% of deaths were attributed to MVP and, of these, the majority (60%) were in women.105
The vast majority of SCD in female athletes are associated with sudden arrhythmic death syndrome where structurally normal hearts are identified on post-mortem analysis.33
Conclusion
In the last four decades, we have witnessed an increasing number of females participating in numerous sporting disciplines at elite levels. Females show similar qualitative adaptations to exercise as men but they have a lower prevalence of concentric LVH and cardiac dimensions exceeding predicted upper limits.
In contrast with men, female endurance athletes do not show a higher prevalence of AF and raised CAC, according to current data. SCD during sport is significantly less common in women than men and is mainly secondary to electrical disease rather than structural abnormalities.
There are still major gaps in our knowledge relating to several CVDs beyond the scope of this review. Further large studies are required in female athletes with a variety of cardiovascular conditions to understand the disease process in our sex.