The recording of intracardiac electrograms (EGMs) in humans was first reported in 1945.1 Since that time, it has been understood that the diagnosis and ablation of cardiac arrhythmias is facilitated by accurate and precise visualisation of those EGMs and the ability to assess the relative timing and direction of electrical propagation within the heart.2 The information contained in intracardiac EGMs is highly dependent on the recording technique and equipment. Unipolar EGMs can provide valuable information regarding the local depolarisation sequence and the direction of electrical propagation that cannot be ascertained from individual bipolar recordings.3 However, unipolar signals are rarely used in clinical cases because they are sensitive to far-field sources and frequently impacted by baseline wandering and powerline (50/60 Hz) noise originating from extraneous electrophysiology (EP) laboratory equipment, specifically when referenced to Wilson’s Central Terminal (WCT).4
Furthermore, high output pacing, radiofrequency ablation, and defibrillation artefacts may also obscure electrophysiologic signals. Whereas some artefacts may result from applied software filters, others, such as high amplitude stimulation, may result in hardware saturation and delayed analogue filter recovery time. Specific situations, such as the delivery of radiofrequency energy, defibrillation and high-voltage pulsed field ablation, present a significant challenge to the hardware components of an EP recording system.
The ECGenius® system (CathVision ApS) hardware platform is engineered to minimise noise by providing higher common mode rejection, lower internal noise and significantly higher input range than conventional EP recording systems. The platform has an optimised hardware design for reference signals, such as WCT, that often are exposed to different background noise from the intracardiac signal paths. As a result, notch filtering, which distorts EGMs and impacts their voltage, is rarely – if ever – needed when using the ECGenius system.
The reliance of current EP recording systems on powerline (50 or 60 Hz) notch filtering to minimise noise may itself distort EGMs. In patients undergoing ventricular tachycardia ablation, notch filtering has been shown to decrease local abnormal electrical activity by reducing its amplitude, changing morphology, and shortening duration, potentially contributing to false positives and false negatives.5 Powerline noise presents not only at the fundamental frequency but also at higher harmonic frequencies which produce complex interference patterns. Such higher harmonics pose a challenge for conventional EP recording systems because of their relatively low sampling rates and, as a result, lowpass filters are commonly employed in addition to notch filtering to the detriment of EGM information.
More recently, adaptive noise filters have offered an alternative to conventional notch filtering. Such filters may preserve the information otherwise distorted by notch filtering by dynamically adapting to the observed noise instead of unconditional removal of signal frequencies. However, their performance relies on correct and timely estimation of noise, which is limited by conventional recording system capabilities, such as sampling rate.
The current study reports a comparison of unipolar EGM recordings from a conventional EP recording system and one of two 3D mapping systems with those from a novel system (ECGenius; CathVision) designed to reduce noise without the need for conventional filtering.
Methods
Procedure
Unipolar EGMs were recorded simultaneously from nine consecutive patients undergoing ablation by a single operator at the Institute for Clinical and Experimental Medicine (Prague, Czech Republic) over the course of 1 week in 2020. Each procedure was performed using conventional diagnostic, mapping and ablation catheters. During each procedure, EGMs were recorded simultaneously on both the CathVision ECGenius EP recording system and the CardioLab (GE HealthCare; control system), as well as one of two electroanatomical EP mapping systems (CARTO 3, Biosense Webster or EnSite Precision, Abbott Laboratories). Unipolar EGMs were recorded at the system-specific sample rates of 977 and 2,000 Hz for the control and ECGenius systems, respectively. WCT was used as the unipolar reference and low and high pass filtering between 0.05 and 500 Hz, with and without notch filters in the control system and without notch filtering in the ECGenius system.
Recording Systems
The two recording systems include an EP amplifier, pin-box assembly for catheter connections, surface ECG cable and a data connection cable to a computer with dedicated recording software and monitors. The signals from all catheter electrodes were split into the pin boxes to provide an identical signal to both systems. The amplifier amplifies, filters, and digitises the signals before they are sent to the computer recording system, where the software further processes, records and provides tools for interactive analysis and visualisation.
Analysis
Sixty-second segments of unipolar signals recorded without notch filter from mapping and ablation catheters were analysed in 10-second windows selected at random from the exported data to estimate the spectral power density of powerline noise. In addition, EGMs related to ablation and stimulation events were assessed for evidence of amplifier saturation and signal morphology distortion. Amplifier saturation was noted when the full waveform or pacing stimulation spike could not be visualised with clipping disabled. Signal morphology distortion was defined when a signal morphology was clearly altered by having changed phases or peak count, or markedly reduced amplitude as compared with an unfiltered signal.
The following three characteristics of the control and ECGenius system EGMs were compared: the mean spectral power density of powerline noise within the 49–51 Hz band estimated using the Welch method; signal saturation; and signal morphology. The frequency of signal saturation among patients was compared between the systems using two-sample test of proportions. In addition, the operator evaluated the compatibility of the ECGenius with the two 3D mapping systems and the associated diagnostic and ablation catheters.
Results
The nine patients included two women and seven men (mean age 66 ± 13.8 years). The patients underwent radiofrequency or cryotherapeutic catheter ablation for AF (five patients), atrioventricular nodal re-entrant tachycardia (three patients) or ventricular tachycardia in structural heart disease (one patient). All nine patients were in normal sinus rhythm at the time of the recordings.
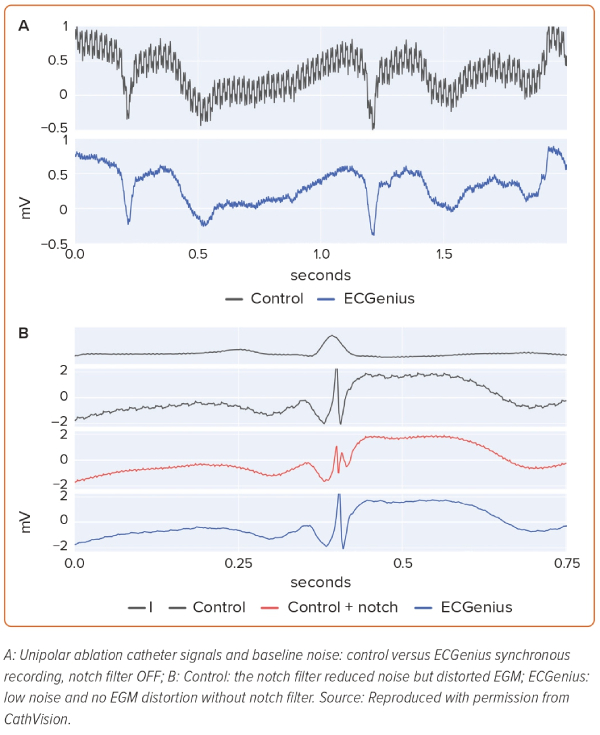
In recordings without the notch filter, powerline noise was visually more apparent on unipolar control system channels than it was on ECGenius system channels (Figure 1A). The mean ECGenius system noise spectral power between 49 and 51 Hz was 6.1 ± 6.2 times lower than that of the control system. Hardware saturation artefact following pacing was observed in eight of the control system recordings and none of the ECGenius system recordings (p<0.001). The mean duration of signal saturation following the pacing spike was 97 ± 85 ms.
Notch-filter-induced EGM morphology changes (reduced amplitude, phase distortion and artificial peaks) were observed in EGMs from five control system recordings, while no notch filter was used on the ECGenius system unipolar recordings (Figure 1B).
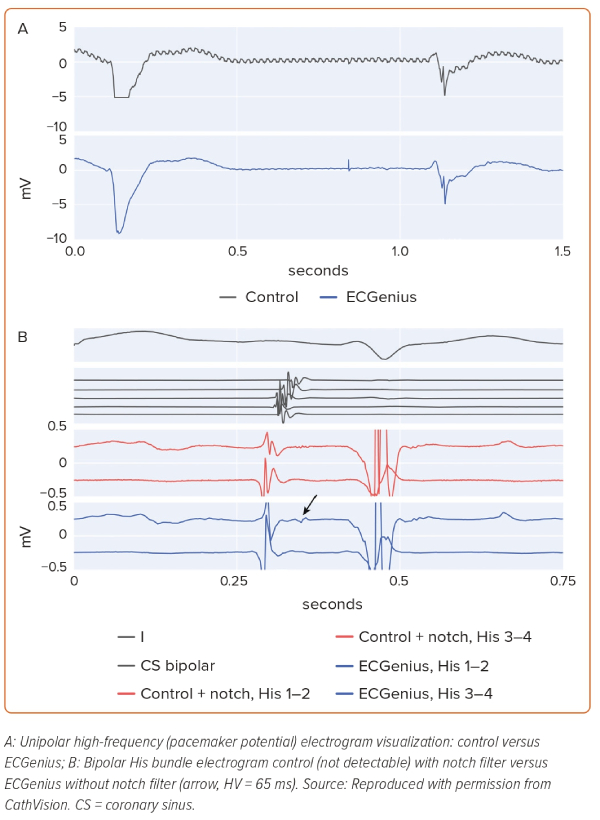
High frequency, low amplitude signals (His bundle EGM, pacemaker spikes, etc.), readily apparent on unfiltered ECGenius system unipolar recordings, were not apparent on unfiltered control system unipolar recordings, likely because of the presence of powerline noise (Figure 2A). Furthermore, notch filtering on the control recording system obscured low voltage His bundle EGMs that were observable on the ECGenius system recordings without such filtering (Figure 2B).
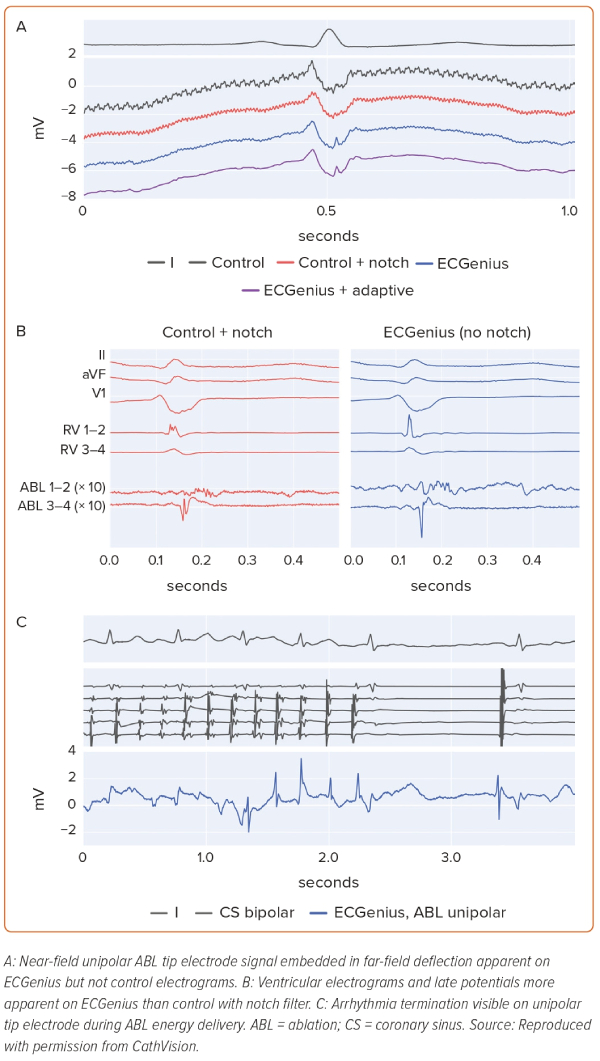
In the patient undergoing ventricular tachycardia ablation, low voltage (<0.5 mV) embedded ventricular EGMs were visualised on ECGenius recordings that were not present on conventional system recordings (Figure 3A). Late potential amplitude was greater, and the potentials were visualised more readily with the ECGenius system than with the conventional system (Figure 3B). Furthermore, ventricular EGM distortion due to notch filtering on the conventional system was not present on the ECGenius system recordings. In no case were EGMs visualised on conventional system recordings that were not seen on the ECGenius system recordings.
During radiofrequency ablation, EGM signal saturation was observed in five of seven control recordings and in zero of seven ECGenius recordings. Importantly, there was no signal saturation in the ablation catheter tip EGM channel in the ECGenius system during radiofrequency ablation. Arrhythmia termination could be observed during energy delivery in the ECGenius system recording (Figure 3C).
The ECGenius system was compatible with both conventional 3D mapping systems (CARTO 3 and EnSite) and commercially available catheters. The EP laboratory personnel reported no issues connecting and using the ECGenius system with either mapping system. The operator reported no instances of the ECGenius system causing noise, artefacts or impacting the signals or maps of either mapping system. Similarly, neither mapping system was observed to impact the ECGenius system performance.
In the EGMs where notch-filtering induced morphology changes or impaired identification of low-voltage potentials, post hoc adaptive filtering was performed and those EGMs were compared with the unfiltered control and ECGenius system recordings. In all cases, the adaptive filter preserved the EGM information while improving the noise floor (Figure 3A).
The adaptive filter could not be applied to data from the control system without modification since the lower sampling frequency was not sufficient for the filter to estimate the powerline noise. After increasing the sampling frequency by resampling with interpolation, it was possible to apply the filter, but it did not significantly improve the noise floor comparable to the unfiltered ECGenius system recordings.
Discussion
In the current study, the unfiltered CathVision ECGenius EP recording system unipolar EGM recordings were found to have a noise power level that was over six times lower than those of the control EP recording system (CardioLab). EGM saturation occurred during radiofrequency ablation in every control recording but in none of the ECGenius system recordings. As has been reported in previous studies, power line notch filtering was used to mitigate 50 Hz noise-distorted EGMs in over 50% of control system recordings.5 The absence of such noise in ECGenius system recordings eliminated the need to use notch filtering and the accompanying EGM distortion. Indeed, the absence of ECGenius system noise enabled visualisation of otherwise obscured EGMs, non-distorted EGMs, and the use of unipolar EGMs.
Unipolar EGMs can provide information regarding both the direction of electrical propagation and the timing of electrical activation that cannot be accurately discerned from bipolar EGMs.6 However, as seen in this study, the powerline noise that is introduced by AC power inherent to EP laboratories can obscure high frequency, low voltage EGMs that may provide important diagnostic information and have important implications for therapy. Furthermore, the notch filtering required to minimise noise when using conventional EP recording systems has the potential to decrease EGM amplitude and affect morphology. EGM morphologic changes may impact analysis of EGM onset and, thus, the interpretation of electrical activation sequence.
Notch filters attenuate a predefined narrow frequency range in the signal, typically with complete attenuation at a central frequency and, in some implementations, the filters may be simultaneously applied at harmonic frequencies for additional powerline attenuation.7 In both situations, physiological EGM deflections in that frequency range will be lost, including sharp near-field potentials. A typical physiological EGM activation may comprise a range of frequencies and the removal of a narrow band will change the appearance of the activation, as seen in this study. In both Figures 1B and 2B it is apparent that the notch filter applied on the control system data alters the activation to appear tri-phasic with reduced voltage. Morphological features such as activation peak voltage and the steepest downslope are affected, which could lead to incorrect annotations of activation timing, potentially affecting the accuracy of activation time mapping techniques.
Adaptive filters differ from notch filters in that they may track the actual frequency and relative strength of powerline interference, thus potentially allowing attenuation of noise with little or no effect on the physiological EGM signal. A post hoc analysis of an adaptive filter applied to the ECGenius system recordings showed no changes in the morphology of critical waveforms. A similar analysis of the adaptive filter on the resampled control system recordings did not show significant improvements in the noise floor. This is likely because of lack of adequate representation of the higher harmonic noise components in the resampled data, which, despite resampling, retains the limited bandwidth of the original recorded data.
During radiofrequency ablation, EGMs recorded by the control system were frequently saturated at the ablation site and at other sites in the chamber where ablation was applied. However, whereas radiofrequency-associated baseline deflection was observed in EGMs from the unipolar ablation tip recorded by the ECGenius system, it did not at any time saturate the system. The absence of tip electrode noise during energy delivery could allow for baseline tracking algorithms to continue to function and the continuous display of ablation EGMs throughout the RF delivery.
In turn, the absence of such noise and saturation may enable the operator to identify therapeutic efficacy (arrhythmia termination, the disappearance of a pre-existing EGM or potential or a change in the surface ECG appearance) during radiofrequency energy delivery. The ability to view EGMs during ablation may also reduce the risk for complications (heart block, etc.) by expediting their diagnosis and enhancing the ability to monitor ablation catheter tip stability.8
Low-voltage EGMs have the potential to convey important information in several circumstances. Electrical conduction through scarred or otherwise damaged tissue may be slowed and result in low-voltage EGMs. Such areas may guide ablation in patients with ventricular tachycardia or AF.9,10 In patients with ventricular tachycardia, a low voltage late potential observed either during sinus rhythm or during the arrhythmia may identify an isthmus and area of slow conduction in the re-entrant circuit.11 Similarly, in patients who have undergone ablation to achieve pulmonary vein isolation, a low-voltage EGM can aid in the identification of a critical isthmus where conduction remains or has recurred between the vein and the left atrial myocardium.12
Analysis of fragmented or dispersed EGMs is essential for several published and commercial methods of persistent AF substrate mapping. Such EGMs may have high-frequency components that overlap the spectrum that is impacted by notch filters. As a result, notch filtering may attenuate transient deflections and induce false potential phases that impact both EGM amplitude and fidelity, ultimately compromising interpretation (Figure 2B).
In recent years, there has been growing interest in the application of machine learning and artificial intelligence (AI) to automate and enhance the mapping of complex arrhythmia mechanisms, such as those responsible for persistent AF.13,14 One approach to training machine learning algorithms is to label EGMs based on their morphological features, such as fractionation. Many of these algorithms are trained using previously recorded stored EGM data. For algorithms that classify EGM morphology, filtering-induced morphologic distortion may cause the algorithm to falsely label EGMs, thereby introducing analysis bias that leads to incorrect conclusions. In effect, the clinical performance of such algorithms may not be reproducible in clinical environments with different noise and filtering conditions. Many EP recording systems, including the control system, apply irreversible user-defined filtering to the stored EGMs. Therefore, these data will be subject to the aforementioned false labelling and bias issues and may not be optimal for training AI systems.15 In contrast, the ECGenius system allows the user to store unfiltered unipolar traces from which any bipolar or AI-optimised filtering can be applied consistently. In the future, the availability of databases of unfiltered EGMs may preserve information that is clinically important and enable improved artificial intelligence and machine learning systems.
Finally, it should be noted that the ECGenius system performed well with both the CARTO 3 and EnSite Precision mapping systems. No issues were reported regarding setting up or using the systems with one another.
Study Limitations
Although the reported findings may stem from technical differences between the compared systems, several limitations of the current study should be considered. The study is limited in scope to assess only EGMs and not clinical outcomes. The clinical significance of the improved signal quality and sensitivity to low-voltage signals remains to be investigated. In addition, the study includes only a small number of patients, and all cases were performed by operators at a single centre. Noise levels may vary across laboratory environments and within a laboratory between the setups for different patients. This may influence the measurement of relative performance among compared systems. Furthermore, the significance of absolute noise levels may vary with the experience of the operator and the nature of the signals that are of interest in a particular case.
The ECGenius system was not used as the primary recording system during the cases. Therefore, while easier identification of signals of interest could have had an impact on procedure time and cost, this study does not allow for that analysis to be performed. Finally, the study compares the ECGenius system to only one legacy recording system. Several mapping systems and alternative EP recording systems exist with different software and hardware specifications and, although some of the current findings are fundamental to common filtering techniques used across systems, a systematic comparison across multiple systems remains to be investigated.
Conclusion
The findings of our study suggest that ECGenius system recordings exhibited less noise and fewer signal artefacts than the conventional control system. Notch filtering that distorted EGMs on control recordings was not required. The novel recording system provided superior EGM data not apparent with conventional systems. Studies in larger groups of patients to further evaluate these findings are desirable. 
Clinical Perspective
- The CathVision ECGenius system electrogram (EGM) recordings were associated with fewer noise and signal artefacts than the conventional system in all nine patients tested.
- In addition, the notch filtering required to reduce conventional system noise distorted EGMs, causing EGM fractionation that was not present on unfiltered ECGenius unipolar EGMs in each of the nine patients.
- The CathVision ECGenius is a novel electrophysiology recording system that provides superior EGM detail not apparent with conventional systems.