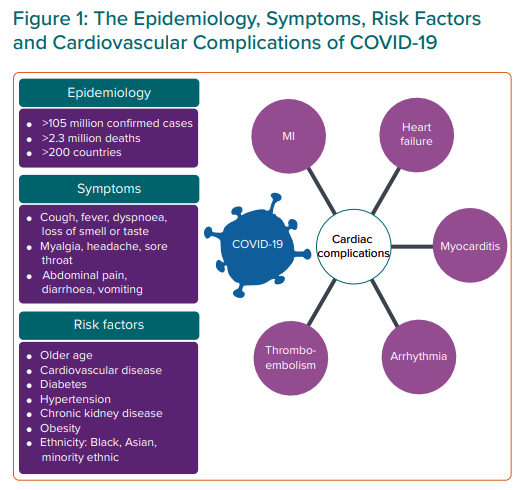
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the pathogen responsible for a series of cases of pneumonia in the Chinese province of Hubei, Wuhan.1 This became known as coronavirus disease 2019 (COVID-19). On 11 March 2020, the WHO declared COVID-19 a pandemic after numerous cases and deaths in more than 100 different countries.2 Globally, as of 7 February 2021, confirmed cases reached 105,394,301 (35,481,004 in Europe) with 2,302,302 deaths.3
The activation of the surface spike protein of SARS-CoV-2 allows it to bind to the human angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 is expressed in the lung and appears the main site of entry for the virus.4 ACE2 is also expressed in the heart, vascular endothelium, kidneys and intestinal epithelium, thus providing a potential mechanism for the multiorgan effects of COVID-19.5,6
The main symptoms of COVID-19 are fever, a new persistent cough and a loss or change to sense of smell or taste.7 There is a long list of other reported symptoms, including myalgia and diarrhoea.8
Cardiovascular manifestations of COVID-19 include MI, heart failure and myocarditis (Figure 1).9–15 Baseline blood tests and common cardiac investigations will help to diagnose these pathologies. Under initial lockdown measures, elite sports were halted but as the pandemic has progressed and plans are made for a ‘new normal’, consensus recommendations for cardiac assessment of athletes who may be affected by COVID-19 have been proposed.16–22 The consensus recommendations and preparticipation screening commonly involve an ECG and some also recommend an echocardiogram.
Athletic training regimens can often exceed ‘normal’ physical limits and if continued, cardiovascular adaptation to exercise can occur. These cardiac changes, known as athlete’s heart (AH) may manifest on investigations such as the ECG or echocardiogram and can mimic mild phenotypes of cardiac pathology. As preparticipation sports screening in the COVID-19 era becomes more routine, an understanding of how to differentiate physiological adaptation from pathological change in athletes will aid clinical decision-making.
The objectives of this review are to:
- Describe the main cardiovascular manifestations of COVID-19.
- To discuss the potential overlap – or the ‘grey zone’ – where physiological adaptation due to AH may overlap with mild pathological change.
- Summarise recent consensus algorithms for the cardiac assessment of athletes who may have been affected by
COVID-19. - Highlight international recommendations for the management of athletes with cardiac pathology related to COVID-19.
Severity and General Clinical Manifestations of COVID-19
When assessing athletes with a current or past history of COVID-19, it is important to consider individual demographics and comorbidities which have been shown to confer worse outcomes. It is less likely that younger athletes will have the same risk profile associated with COVID-19 as masters athletes (aged >35 years). While severe illness due to COVID-19 can occur in healthy people irrespective of age, it predominantly occurs with advancing age and in those with multiple comorbidities. Adults of middle and older age are most commonly affected. Studies with hospitalised cohorts have shown that 74–86% are at least 50 years old.1 Advanced age is also associated with increased mortality from COVID-19.23
Ethnicity also plays an important role in prognosis. Death rates in the UK from COVID-19 among people from black, Asian and minority ethnic (BAME) backgrounds have been shown to be higher than their white counterparts.24 In addition, a systematic review has shown that BAME people are at higher risk of acquiring the virus.25 Comorbidities associated with severe COVID-19 illness and mortality include pre-existing cardiovascular disease, diabetes, hypertension, malignancy, chronic lung disease, smoking, obesity and chronic kidney disease.11
General clinical manifestations of COVID-19 from large registry data include: cough (50%), fever (43%), myalgia (36%), headache (34%), dyspnoea (29%), sore throat (20%), diarrhoea (19%) and nausea/vomiting (12%). Loss of smell or taste, abdominal pain and rhinorrhoea were reported in fewer than 10% of cases each.26 With specific reference to athletes, symptoms of breathlessness disproportionate to the level of exercise should prompt a cardiovascular assessment.
There is a wide spectrum of illness as reported by the Chinese Center for Disease Control and Prevention.27 This ranges from mild disease, with no symptoms or mild pneumonia, in 81% of the cohort; severe disease – such as dyspnoea, hypoxia and significant lung changes on imaging – in 14% of the cohort; critical disease – such as shock, respiratory failure, multiorgan failure – in 5% of the cohort. Overall, the case fatality rate was 2.3% with no deaths reported in non-critical cases.
Cardiac Manifestations of COVID-19
The presence of myocardial injury is an important finding in those with COVID-19 and is associated with increased severity of illness as well as worse prognosis.12,28,29 Myocardial injury is defined as the elevation of high sensitivity cardiac troponin (hs-cTn) above the 99th percentile of normal or new echocardiographic/ECG abnormality.12 Ischaemic and non-ischaemic aetiology of increased troponin levels are described below.
MI
MI can occur as a result of systemic inflammatory response syndrome caused by severe viral infections. In COVID-19, severe inflammatory stress leads to a release of circulating cytokines. This may lead to plaque instability with higher risk of rupture and thrombus formation leading to acute coronary syndrome (type 1 acute MI).30
A type 2 MI due to a myocardial mismatch in oxygen demand and supply may also be a complication of viral infections. Hypoxaemia, vasoconstriction and the haemodynamic consequences of sepsis cause this mismatch which leads to myocardial ischaemia. The medical management of MI in the era of COVID-19 is through established pathways.31
Heart Failure
Acute illness caused by COVID-19 may precipitate heart failure in those with pre-existing or undiagnosed heart disease or due to acute myocardial injury, such as coronary artery disease, stress cardiomyopathy and cytokine storm.11,15
Cardiac Arrhythmias
Cardiac dysrhythmias can occur as a sequelae of a primary cardiac pathology, such as MI or a fibrotic substrate due to myocarditis and have also been reported in the setting of viral illness due to hypoxia, inflammatory stress and fever.11
Thromboembolism
Increased risk of thromboembolism in patients with COVID-19 has been reported with a variety of mechanisms postulated including immobility, hypercoagulable status, a propensity for disseminated intravascular coagulopathy and active inflammation.11,12
Myocarditis
Many viruses are able to bind directly to molecular targets in the myocardium to inflict damage via a range of mechanisms.30 Myocarditis is characterised by inflammatory infiltrates and myocardial injury in the absence of an ischaemic cause. Cases of COVID-19-related myocarditis have been reported, with some reviews suggesting that up to 7% of COVID-19 deaths were attributable to myocarditis.14,32 This may be an overestimate, as the clinical diagnosis in most cases is assumed rather than confirmed by myocardial biopsy.
COVID-19 myocarditis may present with a variety of symptoms, including fatigue, chest discomfort or breathlessness. In athletes, symptoms such as fatigue may be more difficult to establish at an earlier stage due to confounding factors such as the return to full participation in sports after a reduced workload.
Typical baseline investigations for those with suspected myocarditis include blood tests and ECG. Imaging investigations which may be undertaken include echocardiography and cardiac MRI (CMR). These main investigations are discussed below:33
- Blood tests: myocarditis often results in elevated levels of inflammatory markers, such as C-reactive protein. Baseline cardiac enzymes such as troponin and N-terminal pro-B-type natriuretic peptide (BNP) can also be elevated.11
- ECG: changes include ST elevation/depression, PR depression and T wave inversion (TWI).
- Echocardiography: echocardiographic features which may be in keeping with myocarditis include the findings of pericardial effusion; ventricular dysfunction (global or regional wall motion abnormality and reduction in ejection fraction. Left ventricular (LV) hypertrophy has been reported in cases of acute myocarditis as a consequence of myocardial oedema.34
- CMR: an important imaging tool in the diagnosis and risk stratification of myocarditis. Late gadolinium enhancement allows detection of fibrosis and has been shown in studies to be an adverse predictor of mortality when present.35
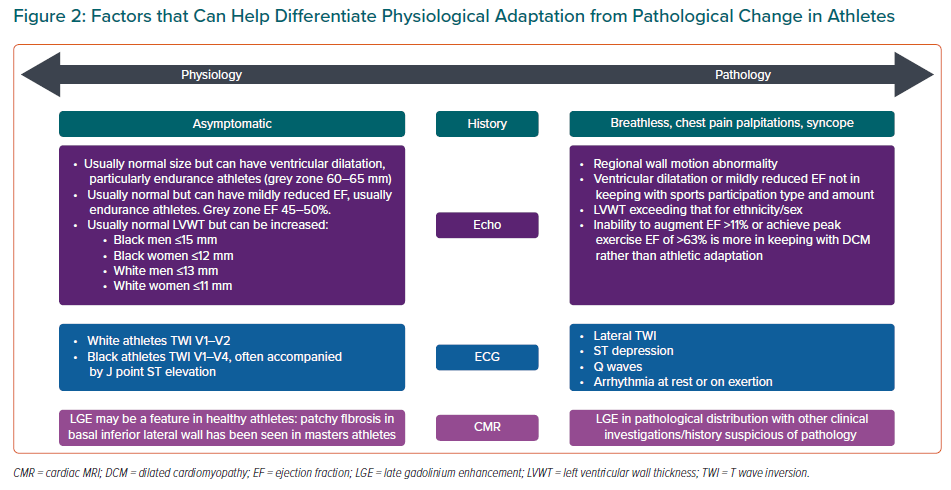
Diagnostic Conundrum in Asymptomatic Athletes: The Grey Zone
An understanding of the ‘grey-zone’ overlap between physiological adaptation and pathology in certain cardiac investigations is important when assessing athletes regarding potential cardiovascular complications of COVID-19. This is particularly relevant during cardiac screening of athletes who have not had COVID-19 symptoms to try to reduce the misdiagnosis of cardiac pathology. Differentiating physiology from pathology with regards to the main investigations used in preparticipation screening is described below and summarised in Figure 2.
Troponin Levels
A considerable proportion of patients hospitalised with COVID-19 have been found to have elevated troponin levels, with some reports finding this in up to 28% of affected cases.30 Care should be taken when interpreting troponin levels in asymptomatic athletes if they have recently participated in sport or training.
Several studies have reported transient troponin elevation after marathons, triathlon events, cycling and other forms of physical activity. Exercise intensity and duration cannot reliably predict the magnitude of troponin release. Cardiac biomarker alterations have been described following table tennis games and prolonged walking.36 There are reports of troponin release beginning as early as 30 minutes into sustained endurance exercise which may represent a normal response to exercise.37–39
ECG
Repolarisation changes such as TWI may be associated with COVID-19-related cardiovascular complications, such as myocarditis. However, athletic adaptation to exercise is associated with a number of ECG changes, which are also dependant on age, sex, ethnicity and sporting discipline.40–44 As a general rule men, black athletes and athletes participating in high endurance sport tend to exhibit more profound ECG changes.
Studies assessing physiological ECG changes have shown that TWI can be normal in white athletes when seen in leads V1–V2.42,44 This is more commonly seen in women (4.3% versus 1.4% in men) and more prevalent in athletes when compared to healthy sedentary controls in women (6.5% versus 3.8%) and men (2.1% versus 1.1%).
Black athletes are recognised to have greater prevalence of TWI than white athletes. In leads V1–V4 this may be interpreted as an ethnically determined normal physiological response to exercise.42 Papadakis et al. described ECG findings in 904 black athletes and compared them with 1,819 white athletes, 119 black controls and 52 black patients with hypertrophic cardiomyopathy.45 TWI was seen in 22.8% of black athletes, predominantly confined to leads V1–V4, often with preceding J-point elevation and convex ST elevation (12.7%).
Sheikh et al. compared ECGs of black and white adolescent athletes and showed a higher prevalence of TWI in the black athletes (V1–V4, 14.3%).40 More recently, Mango et al. raised awareness that premature ventricular beats occurred more frequently in low QRS voltage among Olympic athletes.46 Such anomalies may be suggestive of underlying pathology particularly in subtle cases of myocarditis.
Echocardiogram
Left Ventricular Dilatation
LV dilatation and dysfunction may be a feature of cardiac pathology, such as heart failure or myocarditis, due to COVID-19. Overlap between physiological change and a pathological process may exist in a subset of athletes, particularly endurance athletes. This assessment of LV cavity size has been shown to be increased in athletes when compared to healthy controls.47 While athletes may demonstrate increased cavity dimensions, it is unusual for proportions to reach the pathological threshold, for example with dilated cardiomyopathy (DCM). However, studies have reported that cavity dilatation >60 mm can be seen in up to 14% of male participants. This level of dilatation is more commonly seen in high capacity endurance sports, such as cycling.47,48
As with male athletes, it is unusual for LV cavity dimensions in female athletes to exceed normal limits. Pelliccia et al. studied 600 elite female athletes and found that an LV end diastolic dimension >54 mm was seen in 8% and >60 mm seen in 1%.49 Again, those with greater LV cavity diameter were more likely to be endurance athletes.
Left Ventricular Ejection Fraction
Physiological adaptation may cause some athletes to exhibit a reduced ejection fraction that overlaps with pathological LV systolic dysfunction (ejection fraction 45–49%). This occurs in a minority of athletes, particularly from endurance sports. Abergel et al. showed that in a group of 286 elite cyclists, 147 (51.4%) demonstrated LV end diastolic dilatation (>60 mm) and of these 17 (11.6%) demonstrated reduced LVEF (<52%).50 More recently, Millar et al. reported that an improvement of 11% in ejection fraction from baseline using exercise stress echocardiography has the greatest discriminatory value in differentiating between grey-zone athletes and asymptomatic patients with DCM.51
Left Ventricular Wall Thickness
LV hypertrophy is a rare complication of acute myocarditis.52 Knowledge of physiological hypertrophy in athletes will be of use in differentiating physiological from pathological hypertrophy. Studies have shown that black male athletes have a greater mean maximal wall thickness than white athletes (11.3 mm versus 10 mm). It is uncommon for athletes to exceed the upper limit of normal wall thickness (12 mm in men and 11 mm in women).53 It is more common to see increased wall thickness in black athletes when compared to white athletes: LV wall thickness (LVWT) >12 mm (18% black male athletes versus 4% white male athletes) and >15 mm (3% black male athletes versus 0% white male athletes). Physiological adaptation rarely causes LVWT >16 mm, irrespective of ethnicity.45,54,55A similar finding of increased LVWT is seen in black female athletes when compared to white female athletes. Studies have demonstrated LVWT >11 mm in 3% of black female athletes versus none in white female athletes (with no female athlete exceeding an LVWT of 13 mm).56–58
Cardiac MRI
CMR allows advanced assessment of tissue characterisation in addition to ventricular volume and function assessment. The presence of late gadolinium enhancement (LGE), indicating myocardial fibrosis, has been shown to be a predictor of mortality in those with myocarditis irrespective of ejection fraction.59,60 Normal CMR in those with suspected myocarditis correspond to a low annual major adverse event rate of 0.8% and a death rate of 0.3%.61 LGE distribution in acute myocarditis has been documented to predominantly have a subepicardial or mid-myocardial distribution.62 Several studies have also reported myocardial fibrosis detected by LGE on CMR in athletes, with cohorts of master athletes showing patterns of non-ischaemic fibrosis.63–65 Further studies are needed to assess whether this finding in athletes represents a pathological manifestation of cardiac disease or a reflection of physiological stresses due to prolonged bouts of sports participation.
Returning to Sport Post COVID-19: Guidance
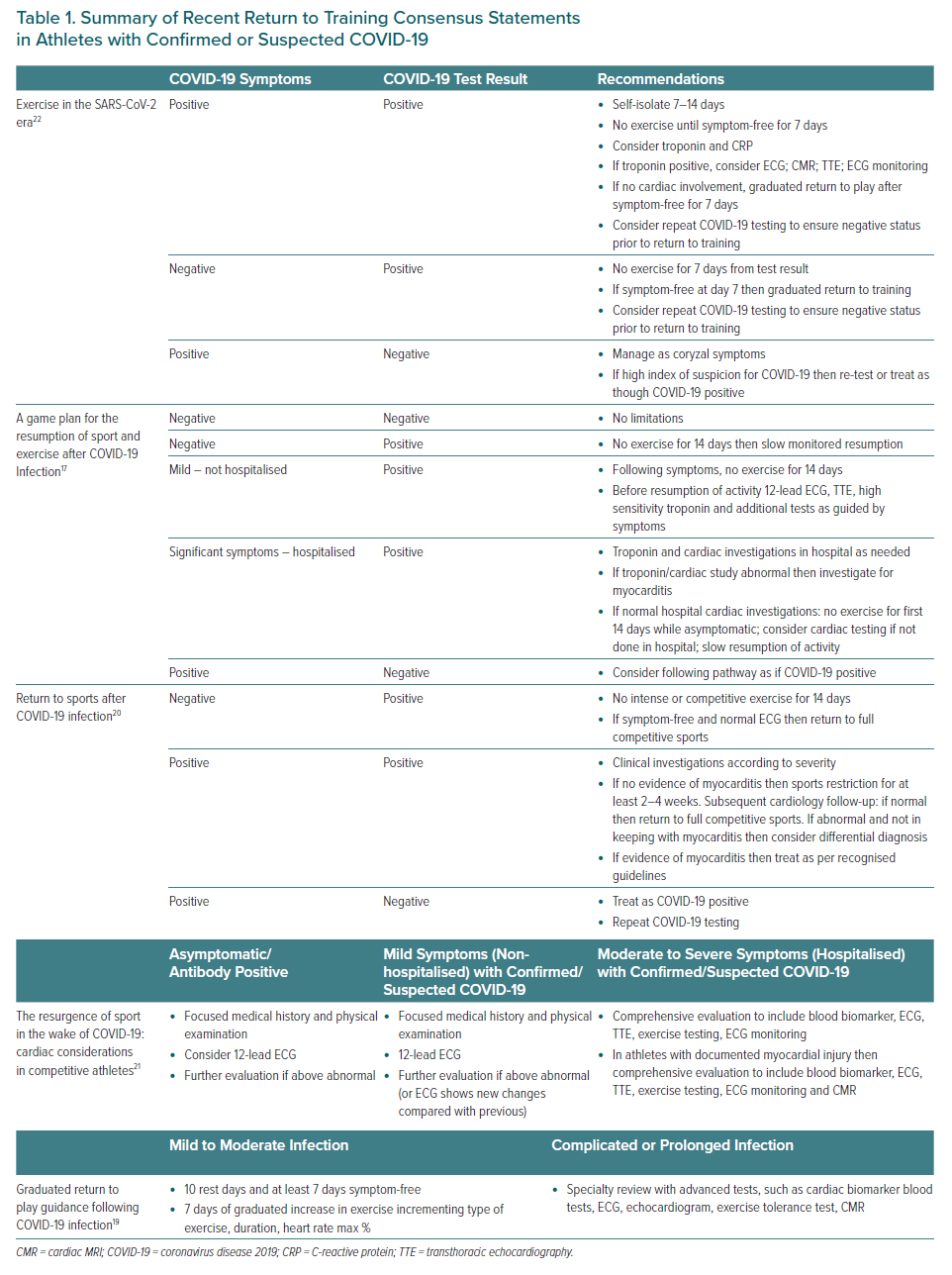
Current recommendations for a return to sport following COVID-19 infection are based on consensus. Some have advocated no participation in intense exercise or competitive sports for 14 days in those who are asymptomatic with a positive COVID-19 test and a normal ECG.20 The use of biomarkers, such as high sensitivity troponin, in asymptomatic people has also been recommended by some before a return to full training.18 A recent graduated return to play consensus statement describes implementation of a protocol following a 10-day rest period (with at least a 7-day symptom-free period).19 Here, activity duration, heart rate percentage and type of exercise is described with a goal of returning to normal training by day 7. It is widely agreed that athletes who have a complicated COVID-19 illness with cardiac symptoms or who have abnormal cardiac biomarker results require a more detailed cardiac assessment including ambulatory ECG monitoring, echocardiography and CMR.16–20 A summary of recent consensus guidelines on return to sports following confirmed or suspected COVID-19 is shown in Table 1.
Cardiac Complications of COVID-19 in Athletes: Returning to Sport
Myocarditis:66
- Consensus guidelines from the European Association of Preventative Cardiology (EAPC) were published in 2019, followed most recently by the European Society of Cardiology’s updated guidelines.67
- Exercise programmes should be restricted for 3–6 months.
- Resumption of training can be considered when LV systolic function has returned to normal range, serum biomarkers of myocardial injury have normalised and clinically relevant arrhythmias are absent on 24-hour ECG monitoring and exercise test.
- Asymptomatic athletes with LGE should remain under annual clinical surveillance.
Pericarditis:66
- Athletes with pericarditis should not participate in competitive sports during the acute phase and can return following complete resolution of the active disease. Three months is considered an appropriate time period to ensure complete clinical and biochemical resolution but shorter periods of at least 1 month may be considered in selected mild cases that have prompt resolution.
- Return to play is reasonable if serum biomarkers, LV function and rhythm monitoring (either 24-hour ECG or exercise ECG) are normal.
- Athletes with myocardial involvement should be treated with recommendations for myocarditis.
- Asymptomatic athletes with small pericardial effusion detected incidentally by imaging but without myopericarditis should not be restricted from sports participation. Periodic surveillance is advisable.
Left ventricular dysfunction:66
- Athletes with DCM and mild LV dysfunction (EF ≥ 40%) who are asymptomatic or do not have a history of syncope or complex rhythm disturbance may compete in sports, with the exception of those where syncope may be associated with serious harm or death, such as motor racing, scuba diving and rock climbing.
- Athletes with DCM should be advised not to engage in competitive sports if they are symptomatic or have one of the following: LV ejection fraction <40%; extensive LGE on CMR; frequent complex ventricular tachyarrhythmias on monitoring or a history of unexplained syncope.
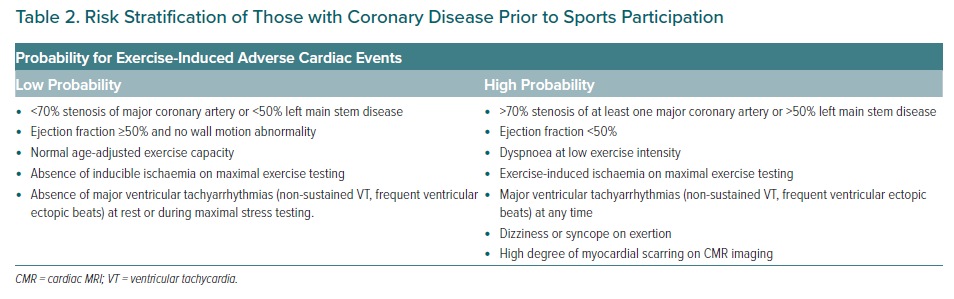
MI:68
- Athletes with proven coronary artery disease as documented by an earlier clinical event, CT scan or coronary angiography should be assessed on an individual basis. The aim is to risk stratify the likelihood of an exercise-induced cardiac event (Table 2). Athletes considered as having a low probability for cardiac events are eligible for most sports although restrictions may apply on an individual basis for certain sports with the highest cardiovascular demand, such as extreme power and endurance disciplines.
Conclusion
This review has highlighted recent consensus statements for the return to sports participation following confirmed or suspected COVID-19.
An understanding of the cardiovascular complications of COVID-19 together with an overview of investigation findings that can help differentiate physiology from pathology in athletes will benefit clinical decision-making.