Coronaviruses are a group of viruses that cause mild to moderate upper respiratory tract disease in humans. In late 2019, a new strain, severe acute respiratory syndrome coronavirus (SARS-CoV-2), caused COVID-19.1,2 By the end of 2022, COVID-19 has been the reason for over 6.6 million deaths and over 600 million infections.3
The most commonly reported symptoms of the infection include fever, cough, difficulty breathing, fatigue and loss of smell or taste.4 However, the pulmonary involvement can lead to multi-organ failure and septic shock. The WHO classified the outbreak as a pandemic on 11 March 2020.5 So far, there is no approved treatment for COVID-19, but the recommended therapies for severely ill and critical patients include corticosteroids, tocilizumab and sarilumab.6
Regardless, the best defence is vaccination. More than 11.8 billion doses have been administered worldwide, with 68% of the global population having received at least one dose.7 The most widely used vaccine is Oxford-AstraZeneca, followed by Pfizer-BioNTech and Moderna’s mRNA vaccines.7,8 All of the approved COVID-19 vaccines have shown excellent efficacy and safety profiles, with a range of mild side-effects, such as pain and swelling at the site of injection, fever and fatigue.9,10 However, previous studies suggested that vaccines could lead to an increased risk of cardiac events.11–13
Several cardiac manifestations such as myocarditis, pericarditis, acute coronary syndrome and arrhythmias following COVID-19 vaccination have been described in case reports and case series frameworks.13,14 The first case report to link myocarditis to COVID-19 mRNA vaccines was published in March 2021, and since then it has been a challenge to understand new emerging cardiac adverse reactions to the novel COVID-19 vaccines.14
In this systematic review, we summarise the reported cases of post-COVID-19 vaccination cardiac disease to determine the susceptible characteristics, outcomes and similarities regarding predispositions. This will provide clinicians with more insight into the prognosis and severity of cardiac conditions.
Methods
We followed the PRISMA and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines during the preparation of our review.15 All of the steps of our study were prespecified, and the protocol was registered on PROSPERO (CRD42022337589).
Study Eligibility
We included all case reports and case series studies if they met the following criteria:
- involved patients with any type of cardiovascular disease associated with any type of COVID-19 vaccine;
- reported that COVID-19 vaccine was a risk factor or indicator for cardiovascular disease;
- assessed the following outcomes: final diagnosis of a cardiovascular adverse effect after COVID-19 vaccination, type of treatment used, response to treatment, whether improved or not, and the incidence of side-effects according to type of vaccine.
We excluded case–control or cohort studies, theses, conference abstracts and reviews. In addition, studies that did not use any COVID-19 vaccine and which assessed different outcomes were excluded.
Search Methods for Identification of Studies
We searched for published relevant studies in the following electronic databases: PubMed, Scopus, Cochrane Library and Web of Science from inception until August 2022 using the following query: ((‘myocarditis’ OR ‘pericarditis’ OR ‘myopericarditis’ OR ‘takotsubo’ OR ‘cardio*’ OR ‘vascular’ OR ‘heart’) AND (COVID-19 OR SARS-CoV-2) AND (vaccine*)).
Study Selection
The selection of studies was performed in two steps by two authors independently. The first step was the title and abstract screening of all recorded citations. The second step involved full-text screening of the articles for eligibility in the analysis. Disagreements were resolved by consensus.
Data Extraction and Management
Three reviewers independently extracted the relevant data from the included studies using an online data extraction form for the following: characteristics of study design, characteristics of the study population, risk of bias domains, and study outcomes, including final diagnosis of a cardiovascular adverse effect after COVID-19 vaccination, type of treatment used, response to treatment, whether improved or not, and the incidence of the side-effects according to the type of vaccine. Conflicts were resolved through consensus.
Assessment of the Risk of Bias in Included Studies
We assessed the risk of bias in the included case reports by two authors independently using the Joanna Briggs Institute (JBI) checklist.16 Each case report was evaluated based on the following domains: clarity of patient demographics, clinical history and presentation, diagnostic assessments, treatments, post-treatment condition, adverse events, and takeaway points. To appraise the case series, we used the National Institutes of Health (NIH) Quality Assessment Tool for case series.17 The NIH criteria assess studies based on the following domains: clarity of objectives, clarity of study population and case definitions, whether the cases were consecutive and comparable, whether interventions were described clearly, the reliability and validity of outcome measurement, adequacy of follow-up length, statistical methodology, and whether results were well-described. Discrepancies were resolved by the third author.
Data Synthesis
We conducted descriptive analyses of our data (categorical data, such as side-effects of the vaccines, and the continuous variables, such as the age of vaccinated patients) using SPSS version 28 for Windows. Continuous variables are expressed as mean ± SD and categorical variables are expressed as frequencies and percentages.
Results
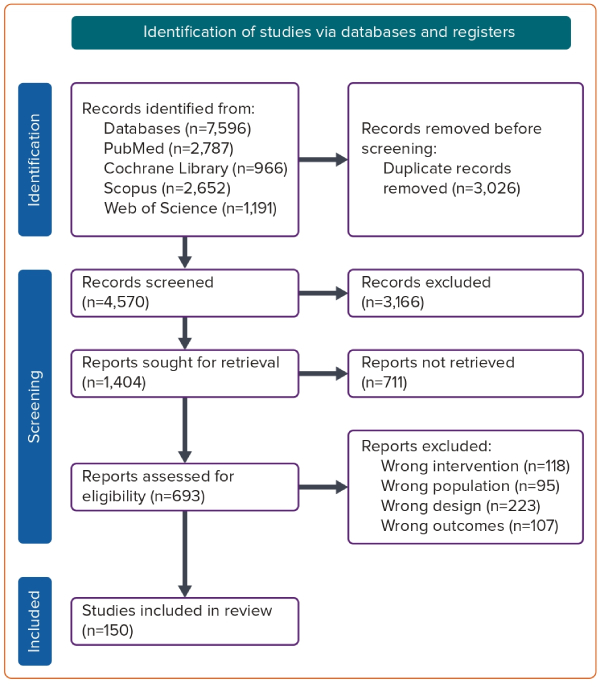
Our search strategy identified 7,596 studies. After removing duplicates, 4,570 abstracts were evaluated, and 693 articles were eligible for full-text screening. During the full-text evaluation, 543 articles were excluded, and 107 were excluded because they met neither the study’s primary nor secondary outcomes. Moreover, we excluded 118 studies because they involved different interventions, 223 articles due to discrepancies in design, and 95 studies because of the variations in population characteristics. Finally, 150 studies were included in our systematic review and meta-analysis (Supplementary Material Figure 1). The PRISMA flow diagram of the study selection process is shown in Figure 1.
In our study, we included case series (n=41) and case reports (n=109) concerning cardiac-related adverse events resulting from the COVID-19 vaccines. The majority of patients were men (n=302, 86.6%), with seven patients each in the 22-year-old and 24-year-old age groups. The oldest patient was 96 years of age and the youngest was 13 years old. Pfizer and Moderna were the most common vaccines (n=255 [72.9%]; n=73 [21.1%], respectively), and more than half of the patients (n=263, 75.4%) had the second dose. In the 150 included studies, the four most commonly reported symptoms were chest pain (140/150 studies, 93.3%) followed by dyspnoea (106/150, 70.6%), cough (39/150, 26%) and fever (30/150, 20%). The average time from vaccination to symptom onset was 94.6 ± 2.1 hours. The patient characteristics, treatment and prognosis in the 150 included studies are listed in Supplementary Material Table 1.
Based on the NIH score, the quality of the evidence in the included case series was good. Of the 41 included case series, 27 of them scored 7–9, representing good quality of evidence, while 12 studies had a fair quality of evidence (score 4–6). The remaining two studies had a poor quality of evidence (score 0–3; Supplementary Material Table 2). The quality of the evidence of the 109 included case reports was assessed as good using the JBI quality assessment tool. A total of 93 of the case reports had good evidence with a quality score >70%, 14 had fair evidence with a score of >50%, and two studies had poor quality evidence with a score of <50% (Supplementary Material Table 3).
Outcomes
Many cardiovascular complications have been reported after vaccination, with the patients who present to hospital having various clinical manifestations. Of 349 patients, 268 (76.6%) had myocarditis, 50 (14.6%) had myopericarditis, 8 (2.3%) had pericarditis, and 4 (1.1%) patients had stress-induced cardiomyopathy. Moreover, 30 (8.6%) and 11 (3.1%) were diagnosed with arrhythmia and ischaemic heart disease, respectively. For the patients with myocarditis, the mean age was 30 years (range 13–80 years). For the patients with myopericarditis, the mean age was 25 years (range 16–88 years). However, the patients with MI presented with a mean age of 65 years (range 42–96 years; Supplementary Material Table 1).
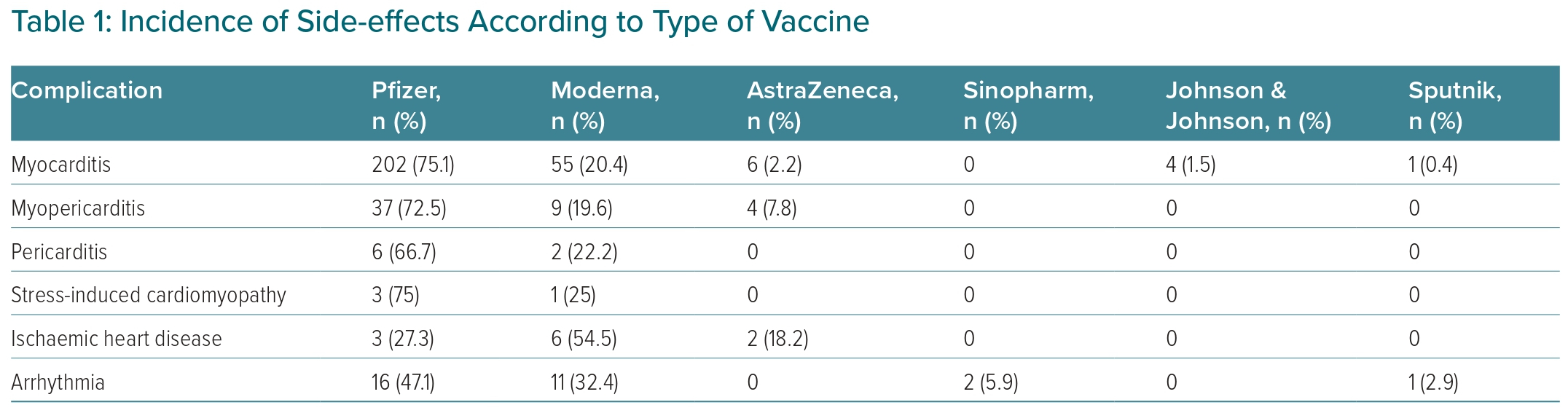
With regard to the incidence of cardiovascular side-effects according to the type of vaccine, 202 (75.1%), 55 (20.4%), 6 (2.2%), 4 (1.5%) and 1 (0.3%) patients who experienced myocarditis had been given the Pfizer, Moderna, AstraZeneca, Johnson & Johnson, and Sputnik vaccines, respectively (Table 1). The most common type of vaccine recorded for myopericarditis was Pfizer in 37 patients (72.5%), followed by Moderna in 9 (19.6%) and AstraZeneca in 4 (7.8%).
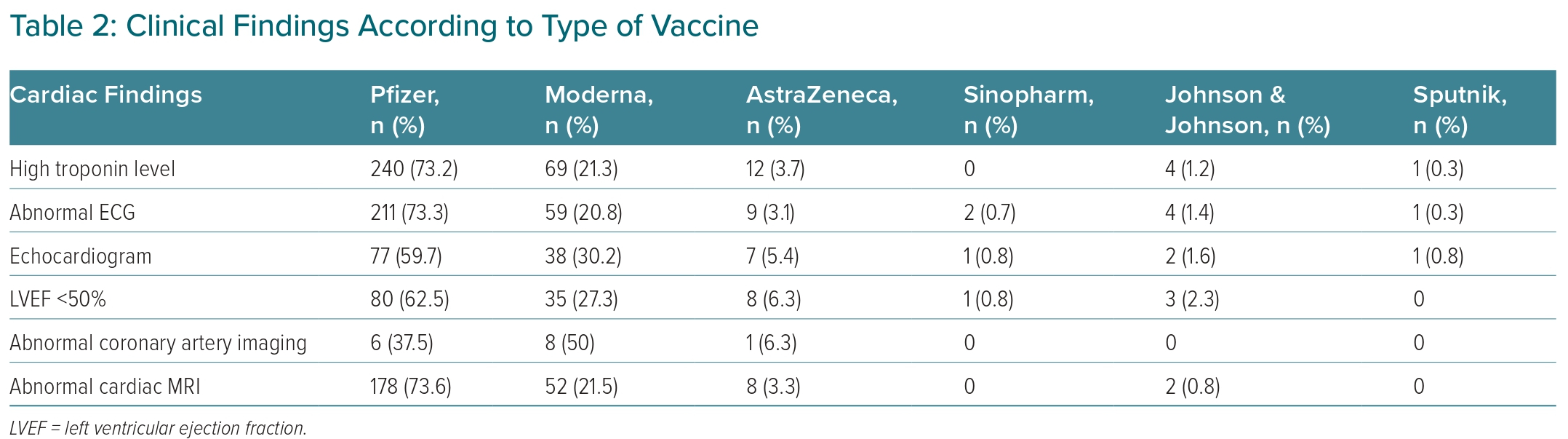
Regarding the cardiovascular investigations, troponin levels were elevated in 326 (93.4%) of the patients. ECG and echocardiogram were abnormal in 286 (82%) and 127 (36.3%) of cases, respectively. Moreover, cardiac MRI was not normal in 240 patients (68.6%), and 15 (4.3%) had abnormal findings on coronary artery imaging. Left ventricular ejection fraction (LVEF) was <50% in 127 patients (36.3%; Table 2). These complications were managed with angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), non-steroidal anti-inflammatory drugs (NSAIDs) and percutaneous coronary intervention (PCI). In almost all cases (n=338, 96.9%), there was an improvement after treatment (Supplementary Material Table 1).
Discussion
Concerns regarding the possible association of adverse cardiac events and COVID-19 vaccination have increased recently.11 The incidence of cardiac disease after vaccination varies depending on demographic features, residual confounding and type of vaccine.11–13 In the present study, we identified a total of 349 patients from 150 published case reports and series (Supplementary Material Table 1). We found that myocarditis was the most common adverse event, followed by myopericarditis, arrhythmia and ischaemic heart disease. Additionally, our analysis revealed that most of the adverse cardiac events were reported after the second dose, and most cases occurred after Pfizer and Moderna vaccinations. When analysed according to country, in the US the most frequently used vaccination was Pfizer, which had been administered in 57.7% of cases involving cardiovascular adverse events. Pfizer was also the most common vaccine in Europe, with complications reported in Germany involving Pfizer in 58.3% of cases. The management of myocarditis and of the other cardiovascular events identified in our study remains primarily supportive and is based on re-establishing haemodynamic stability with the aid of heart failure and arrhythmia treatment. According to our study, most patients requiring treatment were managed with NSAIDs, ACEI, ARBs and PCI, with almost all patients (96.9%) showing marked improvement afterwards (Supplementary Material Table 1).
It has been suggested that molecular mimicry, the interaction between components of the vaccine and specific human proteins that can lead to immune cross-reactivity resulting in autoimmune disease, could be one of the causes of myocarditis.18–21 Variations in hormone signalling could also explain the higher prevalence of myocarditis in the male than in the female population. Testosterone can inhibit anti-inflammatory immune cells while promoting a more aggressive T-helper cell response.19,22 Thus, although it is not clear whether the cardiovascular adverse events following COVID-19 vaccination are associated with or are caused by vaccination, it is clear that they are present and that they should draw the attention of any treating physician.
Generally, the incidence of cardiovascular disease after vaccination varies greatly depending on sex and age. In our systematic review, the mean patient age was 27 years old, with seven patients each in the two most common age groups of 22 and 24 years of age. This age distribution has very important public health implications and justifies further study to better understand why there is a higher prevalence of post-vaccination cardiovascular events in the younger population, especially with regard to myopericarditis and myocarditis, for which the mean patient age was 25 and 30 years old, respectively, compared with the mean patient age for MI, which is 65 years old.
Of note, of the 200 participants under the age of 25, only eight (who also happened to be male) had comorbid lung disease. Our study is the first of its kind to describe these findings in a large sample of patients under 25 years old, a population that generally has no significant comorbidities. In addition, the majority of participants in our review were men. Of these 302 male patients, most had myocarditis. This same pattern of male-dominant prevalence was identified in a study by Ahmed et al. on the development of myocarditis after COVID-19 vaccination.21 In agreement with this, Ling et al. and Chou et al. also noted that young men have a higher incidence of myopericarditis than others receiving mRNA COVID-19 vaccines.23,24 Moreover, the average time from vaccination to onset of symptoms in our review was 94.6 ± 128 hours. This is consistent with previous studies by Pillay et al., Oster et al. and Park et al., in which the onset of symptoms of myocarditis occurred at around 2–4 days after vaccination.25–27
Diagnosis of any cardiovascular event is often based on compatible clinical findings and is confirmed through blood biomarkers and/or ECG findings indicative of cardiac injury, and abnormalities on echocardiography or cardiac MRI. In our review, troponin levels were elevated in 93.4% (n=326) of all cases, consistent with myocardial injury. Of those with elevated troponin levels, the majority (n=240, 73.2%) were patients who had received the Pfizer-BioNTech vaccine, followed by those who had received Moderna (n=69, 21.3%). This can be attributed to the fact that most participants received the Pfizer-BioNTech vaccine, followed by Moderna. These findings of elevated troponin levels are also consistent with reviews by Park et al. and Lee et al. that noted an elevated troponin level in most of the reported cases.27,28
A previous study by Puchalski et al. supports our findings. They reported a case series of COVID-19 vaccination-induced myocarditis in teenagers in which characteristic features of acute myocardial injury, including ST segment changes and repolarisation time abnormalities, were present in all cases.29 Interestingly, our study is the first to report data on MI after vaccination, which occurred in three young adults of 17 years of age. Thus, although the younger generation can have other comorbidities that predispose them to MI, the incidence of MI in younger adults is still unclear. Previous publications have theorised that this increased incidence is due to arterial spasm or subclinical coronary disease aggravated by post-vaccination vasculitis/arteritis.18,19 Arterial spasm can occur due to several factors in young adults, such as cocaine use, alcohol abuse or stress-related adrenergic reaction. These cause not only spasm but also plaque erosion and acute thrombosis.18,19
Furthermore, in our study 127 (36.3%) of the patients had LVEF <50%. As shown by Ahmed et al., compared with patients with COVID-19, patients presenting with post-vaccination cardiovascular disease, especially myocarditis, had a higher LVEF percentage.21 This finding is also consistent with three systematic reviews by Fronza et al., Shiyovich et al. and Behers et al. that investigated myocardial injury after COVID-19 vaccination and found that more than half of the patients had LVEF% >50%.30–32
Finally, we compared our results with seven previous reviews in an attempt to draw a conclusion and represent a broader range of cardiac events after vaccine treatment (Supplementary Material Table 4).21,23,24,27,28,32,33 It is critical to highlight that the causal relationship between the cardiac adverse events and COVID-19 vaccines is not confirmed. Therefore, we recommend further well-designed and high-quality studies with a larger sample size to increase the possibility of providing level 1 evidence with regard to the possible association between cardiac adverse events and COVID-19 vaccines.
Limitations
The major limitations in this review include the lack of cohort and case–control studies due to the quality of the data of these studies and to avoid a reporting bias, which may have a potential impact on the consequences; the low sample size due to the limited number of published cases; and the variation in the definitions of myocarditis and other cardiovascular diseases in the included studies, as well as the diversity of included studies from different countries, institutions and healthcare systems, which may affect the per study outcomes and introduce a degree of uncertainty into the per study results. However, this discrepancy may have little impact on the precision of the findings.
Conclusion
Most of the adverse cardiovascular events were reported after the second dose of COVID-19 vaccination. The most common cardiovascular complications after vaccination were myocarditis, myopericarditis, arrhythmia and ischaemic heart disease. The young population, especially male patients, could be more vulnerable to myocarditis. The majority of patients had marked improvement, as evidenced by their clinical and laboratory findings. Further research is needed to verify the possible association between COVID-19 vaccines and cardiac events.