Cardiovascular disease (CVD) is the primary cause of morbidity and mortality in chronic kidney disease (CKD). CVD mortality risk doubles and triples in CKD stages 3 and 4, respectively.1 This relationship is complex and bidirectional, with each condition increasing the incidence and progression of the other.2,3 Indeed, the heart and kidney are inextricably linked, as exemplified by the cardiorenal syndrome whereby dysfunction of one organ induces and advances dysfunction in the other.4,5
CVD assessment and management are complicated by the presence of CKD and its comorbidities. Additional CKD-related risk factors and alternative pathophysiology also contribute to CKD-CVD.1 CKD patients are largely excluded from clinical studies, resulting in a poor generalisability of assessment tools and a poor evidence base for the safety and efficacy of available treatments. Systematic reviews in 2006 found that CKD patients were excluded from 56–75% of CVD trials. This was even higher for patients with end-stage kidney disease (ESKD).6,7 Updated reviews in 2018 demonstrated continued underrepresentation with 46–57% of CVD studies excluding CKD patients.8,9 Recent recommendations to promote inclusion of CKD patients have considered the role for regulatory and financial incentives, modifications to study design and collaboration between cardiologists and nephrologists. These strategies are needed if we are to obtain the essential data to guide management of this vulnerable population.10 Indeed, current treatment options are associated with a high burden of adverse events, complications and concerns about dose adjustments particularly for renally-excreted medications.1 Clinicians act with caution, resulting in CVD being under-treated in many cases.11 This article aims to address the current understanding of CKD-CVD and the limitations. It will focus on coronary artery disease (CAD), arrhythmias, heart failure (HF) and valvular heart disease (VHD).
Risk Factors and Presentation
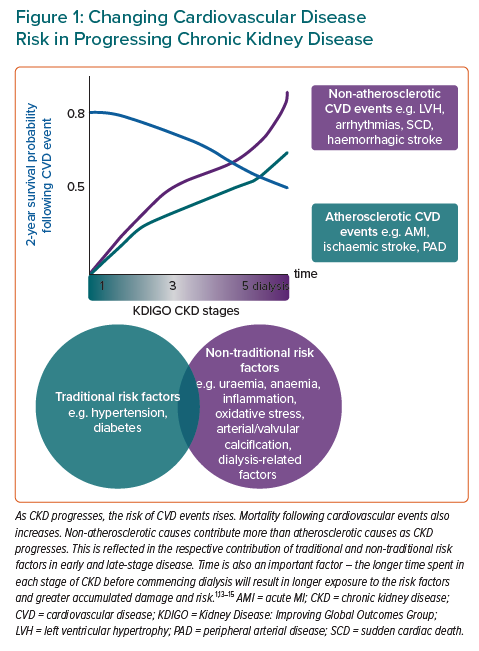
Traditional cardiovascular risk factors, such as hypertension and diabetes, are very common in CKD. Further CKD-related CVD risk factors also contribute, such as uraemia, anaemia, inflammation, oxidative stress and factors related to dialysis.1,12 These CKD-related factors contribute more with a decline in estimated glomerular filtration rate (eGFR). This is reflected in the pathophysiology of CVD in CKD patients as non-atherosclerotic cardiac events becoming increasingly common as CKD progresses (Figure 1).1,13–15
CVD and related events often present atypically in CKD and with fewer symptoms. Only 44% of late-stage CKD patients have classical pain with an acute MI (AMI) compared with preserved renal function.16 These patients more commonly present with AMI than with stable angina.17 They also more commonly present with non-ST segment elevation MI than ST-elevation MI.18 As such, a high clinical suspicion must be maintained.
Assessment
CKD and related variables such as eGFR and albuminuria are rarely included in CVD risk prediction tools, limiting their use for stratification, investigation and management of these patients. Moreover, these tools are believed to underestimate CVD risk in CKD. Recent data-driven evidence has increased utility in early-stage CKD, but generalisability to ESKD remains poor.1,19
Biochemical and radiological investigations to stratify risk among patients are also challenging; investigations may cause adverse effects which affects the interpretation of results. Cardiac biomarkers such as troponins and N-terminal pro B-type natriuretic peptide are frequently raised in CKD, limiting their specificity for cardiac abnormality.1,20,21 Research is needed to elucidate the significance of CKD-specific troponin thresholds and the value of serial measurements for CAD and AMI diagnosis.22
Gold standard radiological investigations including CT angiography and pharmacological MRI stress test may cause contrast-induced nephropathy and nephrogenic systemic sclerosis, respectively and the risks must be balanced against diagnostic significance.23,24 Pharmacological stress echocardiography and nuclear myocardial perfusion scans are more widely available and are not subject to the same risk profiles, however they provide a lower sensitivity and specificity when compared to the gold standard tools, and when used in non-CKD patients.24–26
Coronary Artery Disease
CAD is common in CKD, with incidence rising linearly as eGFR declines. Simultaneous management of CAD is challenging and it is associated with a poor prognosis in these patients.27 The mainstay of CAD management is lipid-lowering medications. Statins become less beneficial with CKD progression, with no clear benefit in dialysis patients. Newer medications are efficacious and safe in mild/moderate CKD, but effects are unclear in advanced disease. The SHARP trial and subsequent studies have argued that concomitant use of statins and ezetimibe may safely attenuate cardiovascular risk even for ESKD, although further investigation is needed.28,29
The alternative management of CAD is revascularisation. Although revascularisation reduces cardiovascular symptoms, it only appears to confer survival benefit in CKD patients with a high baseline cardiovascular risk, and is associated with a higher rate of renal failure.30 The burden of permanent dialysis treatment must therefore be weighed up against the symptomatic benefits of coronary revascularisation.
The ISCHEMIA-CKD trial showed no significant mortality benefit after revascularisation in stable CAD for CKD patients.31 Revascularisation also poses significant risks that must be considered, including contrast-induced acute kidney injury, poor access sites for cardiac catheterisation, post-procedure infection, potential implications for vascular access for dialysis and duration of dual antiplatelet therapy.1 CKD patients are also less likely to receive guideline-recommended treatments for acute coronary syndrome. Risk-benefit analyses are challenging as these patients face a higher risk of ischaemic and haemorrhagic complications, and the mortality benefit of invasive strategies declines with worsening eGFR.20
Heart Failure
About half of all patients with heart failure (49%) have CKD and the combination is associated with greater mortality and hospitalisation.3,32 HF and mortality risk worsen as renal function declines and this is independent of age, duration of HF or diabetes.32 Diagnosis is challenging as symptoms of fluid overload such as dyspnoea and peripheral oedema are common to HF and CKD.21
Pharmacological management for HF with reduced ejection fraction (HFrEF) is efficacious in CKD stages 1–3. However, evidence is sparse for their use in CKD stages 4–5 as these patients are largely excluded from clinical trials. Guidelines support use of renin-angiotensin-aldosterone system inhibiting drugs for CKD-HF, yet they are often underused because of the risk of hyperkalaemia.33 Alternative pharmacological management with diuretics and ß-blockers may cause drug resistance and electrolyte derangement.21 Additionally, optimising CKD-related conditions can alleviate HF. There is a strong evidence base for using IV iron for iron-deficiency anaemia in CKD-HF.34
Notably, empagliflozin has demonstrated efficacy in treatment of HFrEF and HF with preserved ejection fraction, for which there were previously no treatments.35,36 Empagliflozin is a sodium-glucose cotransporter 2 inhibitor that was primarily used in the treatment of type 2 diabetes, a common CKD cause and comorbidity. It has also been shown to slow the rate of renal function decline, regardless of CKD severity.35,37
Arrhythmias
CKD predisposes individuals to arrhythmias, most commonly AF with 16–21% of CKD patients and 15–40% of dialysis patients have AF.38,39 AF also increases risk of CKD and its progression.40 The conditions share many risk factors and unlike numerous CVD risk scores, the AF CHA2DS2-VASc and HAS-BLED scores work similarly in CKD as in the general population.41,42
Managing AF and stroke risk in these patients is challenging due to the safety and efficacy of available treatments. Direct oral anticoagulants (DOAC) are non-inferior to warfarin when creatinine clearance is 30–50 mL/min, and are markedly safer.41,43 However, in later-stage CKD, there is conflicting evidence for using DOACs at adjusted dose and insufficient evidence to support warfarin use.41,44 These patients have a high risk of bleeding and other adverse effects. In these patients, consideration must also be given to the competing risk of death in CKD when assessing risk benefit in AF stroke prevention.45
Sudden Cardiac Death
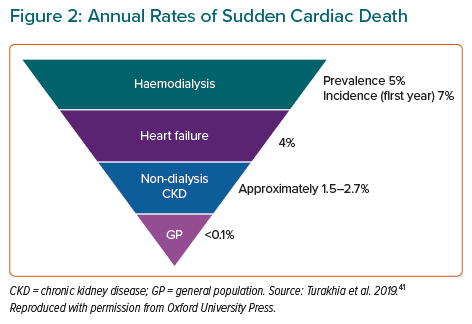
Sudden cardiac death is very common in CKD, particularly in ESKD (Figure 2). Numerous risk factors have been identified, but further research is essential to elucidate the contribution of these risk factors and to facilitate prevention strategies.41
Valvular Heart Disease
VHD is common in CKD and is associated with significantly reduced survival with 5-year mortality with at least mild aortic stenosis (AS) or mitral regurgitation (MR) being >50% greater than people without CKD.46 The primary pathophysiological mechanism is valvular calcification, which is more prevalent than in the general population, worsens with declining renal function and is independently associated with adverse cardiovascular outcomes.47–49 Clinical presentation of VHD can often be mistaken for CKD as symptoms also include dyspnoea and fatigue.
Calcimimetic drugs have been considered for VHD prevention in CKD, but this requires further study. Valvular regurgitation in these patients is often functional and potentially reversible with adjustment of volume status using dialysis. Strict volume status control may prevent the progression of VHD, but no medical intervention has shown benefit.49
Surgical and transcutaneous atrial valve replacement are commonly performed in these patients. These procedures carry similar risks and benefits compared to the general population in CKD stages 1–3. While this improves symptoms throughout CKD, rates of complications, progression to dialysis and mortality risk increase unacceptably with worsening eGFR.50,51
Percutaneous mitral valve replacement with MitraClip is also commonly performed, improving cardiac function, symptoms and renal function in CKD patients regardless of eGFR at baseline. However, these patients face worse outcomes with higher rates of hospitalisation and mortality. Careful patient selection for these interventions is therefore essential. Additionally, consideration of patients’ individual treatment goals, such as symptomatic relief versus life-prolonging treatment, are pivotal to informing risk-benefit analyses and patient-centred shared decision-making.49
The Multidisciplinary Approach
The complex bidirectional relationship between CVD and CKD, combined with common multimorbidity and limitations of standardised risk scores, benefit from corroboration between specialists. A multidisciplinary team approach including cardiologist, nephrologists and allied healthcare professionals improves management and patient-centred HF care through joint clinics (Figure 3).52
Conclusion
CVD is very common in CKD and vice versa. The presence of each condition promotes incidence and progression of the other. Despite the high prevalence, morbidity and mortality of these comorbid conditions, there are significant limitations to our current knowledge and management of this vulnerable group. CKD patients are grossly underrepresented in CVD research, limiting generalisability of available data. Standardised risk scores that are often used to guide investigations and management in CVD are likely to underestimate risk in CKD. Moreover, investigations have poorer sensitivity and specificity and may come with unacceptable adverse effects.
Drug management is complex due to limited evidence, dose adjustments due to renal function and adverse effects. As such, these patients are less likely to receive guideline-recommended management. Interventions are largely safe and effective in CKD stages 1–3, but there is insufficient evidence to support their use in later-stage kidney disease. If interventions are used in CKD stages 4–5, they are more strongly associated with adverse effects and complications.
As CKD progresses and the incidence of CVD rises, we have less knowledge and fewer management options. The complexity of these comorbid conditions necessitates a multidisciplinary approach to improve patient-centred care.