Coronary arteries typically run a subepicardial course; however, they sometimes tunnel intramurally through the myocardium in an anatomic variant known as myocardial bridging (MB). The exact prevalence of MB is unknown. It is seen in 5–58% of autopsies and on average it is present in one-third of all adults.1 The MB rate on coronary CT is similar to the rate in autopsies, whereas the rate on angiography is 0.5–16%.2 The discrepancy between these numbers suggests a possible underreporting of MB when angiography is used as the imaging tool of choice.
In angiography, many factors play a role in the visualisation of the dynamic and phasic narrowing of the artery, namely, the length of the affected segment, the thickness of the myocardium, the orientation of the segment with respect to its surrounding myocardium and the observer’s experience.3
This congenital anomaly typically affects, but is not exclusive to, the mid-segment of the left anterior descending artery (LAD). Less frequently, the diagonal and marginal arteries are affected, in 18% and 40% of cases, respectively.1 The length of the MB segment is typically 10–30 mm and it usually runs at a depth of 1–10 mm.4,5 MB deeper than 5 mm is typically not managed with a myotomy.6 According to a retrospective study in Turkey, the prevalence of MB was higher in men than in women, but the influence of or association with hormones was not explored in that study.7 A similar finding is reported by many prior studies without a correlation between hormone effects and MB.5 Prevalence is also increased in patients with hypertrophic cardiomyopathy (HCM) and in heart transplant recipients.8,9
Myocardial Bridging and Heart Transplantation
Between 33% and 63% of heart transplant recipients have been found to have MB.9,10 Most commonly, the bridges have been found to occur in the LAD. Wymore et al. believe that the stiffness and hypertrophy of the transplanted heart allow for better MB detection on angiography, due to the restrictive haemodynamic pattern that forms after the transplant.9 The theory that a decrease in compliance and an increase in fibrosis is associated with the discovery of a higher incidence of MB, is supported by other studies in which there was a higher incidence of MB in patients with left ventricular hypertrophy (LVH), the hallmark of which is the development of myocardial fibrosis.11,12
Wymore et al. also found that the bridge length and degree of stenosis changed over time. This could have occurred due to the differences in the imaging used over time, but they also proposed that it could be due to the changing status of the myocardium secondary to transplant, wherein fibrosis occurs secondary to therapy with drugs such as cyclosporine, and the stiffness of the transplanted heart increases due to chronic rejection.9
Tanaka et al. conducted a study on MB after heart transplantation and found that heart transplant patients with MB had significant acute proximal atherosclerotic plaque build-up when compared with non-MB heart transplant patients, who had diffuse atherosclerotic build-up in the LAD. On Kaplan–Meier analysis, the presence of MB was associated with low event-free survival after heart transplant.13 Hence, it has been proposed that preoperative screening should be done on all donors before harvesting and implantation, especially in young donors with an unexplained or suspicious cause of death.10,14
Clinically, MB is usually not associated with adverse events. However, the haemodynamics in an individual with MB can be affected, leading to cardiac events. Whether a patient is symptomatic or not will depend on the length and thickness of the segment, the orientation of the MB in respect to the surrounding myocardium and the presence of any adipose or connective tissue.6 Patients with MB have presented with myocardial ischaemia, acute coronary syndrome (ACS) and coronary spasms.15–17 It has also been associated with myocardial stunning, ventricular arrhythmias, takotsubo cardiomyopathy (TCM) and sudden death.2,18–21 MB should always be considered as a differential diagnosis in young patients without any coronary risk factors who present with exertional chest pain or who have anteroseptal perfusion defects.1
Pathophysiological Alterations in Haemodynamics Due to Myocardial Bridging
In autopsies, as well as on intravascular ultrasound (IVUS), the intramural, as well as the distal portion of the MB, have a lower burden of atherosclerotic plaque compared with the proximal portion. It is the proximal segment that is predisposed to plaque formation due to the low wall shear stress and high oscillatory wall-flow. In vessels with these types of shear stress patterns, as in the proximal MB segment, there is an increased expression of vascular cell adhesion molecule 1, intercellular adhesion molecule-1, C-reactive protein, interleukin-6 and reactive oxygen species, leading to a highly pro-atherogenic environment for the development of plaque.22,23 Furthermore, the increased wall tension and stretch may lead to endothelial injury and plaque rupture, which will consequently lead to thrombus formation in the proximal segment.1
Within the bridged segment, it is thought that the high shear stress is protective against the formation of large clinically significant plaque. Masuda et al. showed that tunnelled segments expressed a lower concentration of endothelial nitric oxide synthase (e-NOS), angiotensin-converting enzyme and endothelin-1.24 Additionally, in MB there is a lower tendency to branch off than in other segments, which leads to different haemodynamics.25 The study that showed this, however, did not look further into whether this had a protective role in the vessel.25 A few studies suggest that MB has a pivotal role in causing coronary spasms.26
In studies on the effect of MB on the coronary flow reserve, not only was the systolic component of the artery compressed, but this obstruction also continued into diastole for an average time of 136 ms. There is a continuous delay in early diastolic gain with a reduction in mid-diastolic diameter of >30%.27
Taking this into consideration, it implies that situations with tachycardia will lead to decreased diastolic filling time, which then leads to the amplification of any abnormalities that are occurring during systole.6,27 Using intracoronary Doppler, diastolic flow disturbances were seen to predominate in rapid atrial pacing and there were instances of drops in the systemic arterial pressure or coronary perfusion pressure.27 Individuals with MB are also more likely to present with clinical symptoms if they have concomitant hypertrophy of the left ventricle (LV), increased platelet aggregation, or abnormal vasomotion of the coronaries.27
3D Reconstruction of Myocardial Bridging Using Computational Flow Dynamics
One of the recent advances in the field of biomedical engineering is computational flow dynamics (CFD), which enables reconstruction of a 3D model of the coronary artery using a real-time simulation with a more magnified explanation of haemodynamic variation in the stenosed part of the arterial segments.28
The CFD models show that there is high shear stress in the tunnelled artery without any recirculation zones. The proximal segment of the tunnelled artery has different dynamics wherein there is low shear stress but multiple recirculation zones, which, along with an oscillatory flow pattern, cause blood particles to stay in the vessel longer than usual, thus contributing to vessel remodelling and plaque formation.28 Factors affecting atherosclerotic build-up include disrupted flow patterns (especially flow recirculation, which exacerbates LDL internalisation), cell adhesion and monocyte adhesion to the endothelium.29
The range of recirculatory flow governs the appearance of prothrombotic and atherogenic biomarkers at the bifurcation of arteries. Oxidised LDL uptake, monocyte adhesion and tissue factor expression are increased by up to threefold, while phosphorylated e-NOS and Krüppel-like factor 2 have been shown to decrease by up to twofold in recirculation areas. The endothelial health depends highly on arterial flow patterns, and vessels react differently to various flow types, as seen on 3D flow simulations using CFD. The carotid and coronary artery bifurcations behave similarly in multiple aspects.29
Diagnosis of Myocardial Bridging
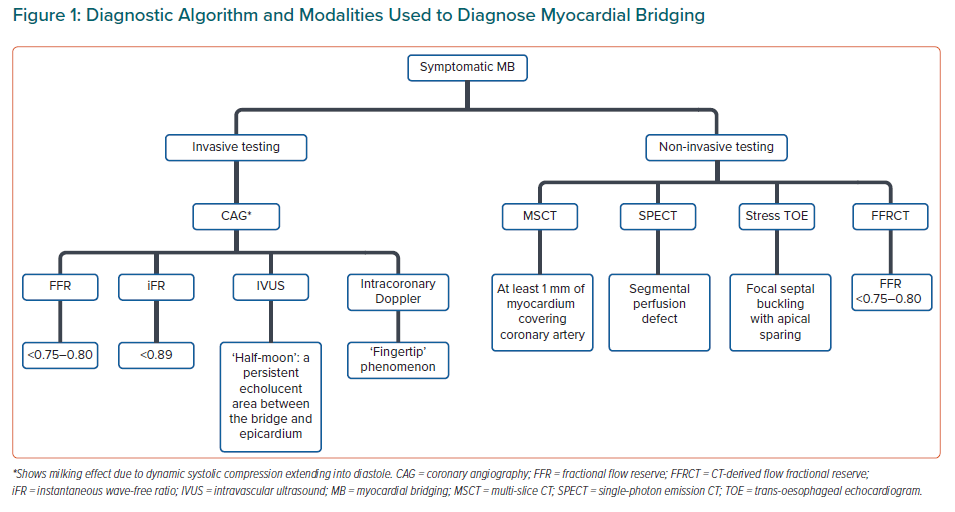
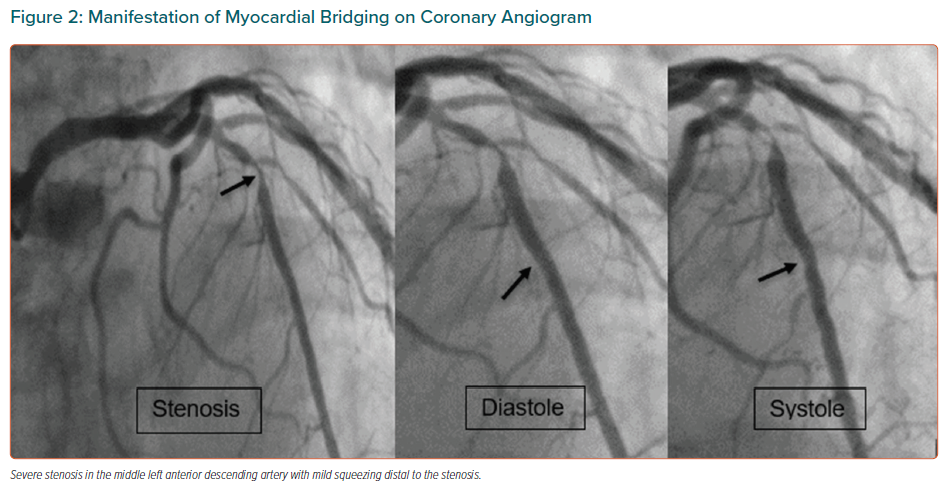
There is no gold standard test for the diagnosis of MB. It can be visualised using both invasive and non-invasive techniques (Figure 1). It is important that if such a diagnosis is made, the haemodynamic effects should also be assessed, using Doppler flow catheters or intracoronary pressure wires.1 On conventional coronary angiography, the first impression of MB is as an intramural course of epicardial arteries (Figure 2).
Tools to Diagnose Ischaemia in a Stenosed Segment
Instantaneous Wave-free Ratio versus Fractional Flow Reserve
Traditionally, fractional flow reserve (FFR) has been considered the gold standard for the measurement of differences of pressure across coronary artery stenosis. However, it has limitations with regards to deciding the need for intervention when the value lies between 0.75 and 0.80. This is known as the FFR grey zone. According to the principle of physiologic autoregulation, for a stenosis to be significantly noticeable, it should affect the diastolic phase. The probability of the decision to revascularise will change by nearly 50% in this grey zone area. The specificity of FFR falls to 80% when compared with non-invasive methods in this grey zone, which calls for attention. Clinicians should personalise the treatment strategy if FFR is in the grey zone.30,31
FFR assumes a directly proportional relationship between pressure and flows in the intracoronary stenosed segment. The measurement of FFR requires a constant flow of blood across the distal stenosis, hence, adenosine (intracoronary vasodilator) is administered to measure FFR throughout the cardiac cycle. FFR is a pressure-based gauging modality to estimate a loss of blood flow based on pressure loss: for example, a 20% pressure loss across the stenosis is equivalent to an FFR value of 0.80.30
During systole, there is high intracoronary resistance and high compression in myocardial vessels and vice versa in diastole. This shows that the nature of resistance is not constant; instead, it is dynamic and varies periodically. The dilating effect of adenosine is an unwelcome experience for many patients because it causes chest heaviness and tightness, can aggravate atrioventricular blockade and in some cases can lead to dyspnoea with hypotensive episodes. All the aforementioned factors limit the usage of FFR for the diagnostic quantification of stenosis. This makes the instantaneous wave-free ratio (iFR) a better option over FFR.31,32
iFR measures the physiological impact of the stenosis on the distal coronary bed using wave intensity analysis. It can separate six kinds of waves that originate in the myocardium at specific points and which are directional in nature. These waves can be categorised, based on their origin, as distal or proximal; and, based on their effect on blood flow, as compressive or expansive. The ideal phase for quantification of stenosis is in diastole, given that it is the wave-free period.30
Two noteworthy clinical trials, iFR-SWEDEHEART (NCT02166736) and DEFINE-FLAIR (NCT02053038) have proven that iFR is non-inferior to FFR in deciding the need for intervention.32 iFR-deferred intervention outcomes were similar to FFR-deferred intervention outcomes. It was found that ACS patients had worse outcomes than stable coronary artery disease patients when FFR was used and had similar outcomes when iFR was used.33 iFR is also the best modality to diagnose a diffuse atherosclerotic disease, to enable clinicians to opt for coronary artery bypass graft (CABG) instead of stent placement.30 FFR values are unreliable due to the dynamic nature of coronary obstruction due to MB. The systolic overshooting and negative systolic pressure gradient (i.e. the average coronary pressure distal to the MB is higher than the aortic pressure during systoler) compromise FFR readings.33 FFR is validated for a fixed stenotic lesion that is not affected by systolic compression at rest or by inotropic challenge.33 In contrast, iFR allows us to anatomically localise and highlight coronary arteries that run intramurally and to locate points of pressure drops (tunnelled segments) during the cycle.33
Non-invasive Myocardial Bridging Assessment
Non-invasive techniques to assess MB include multi-slice CT (MSCT), single-photon emission CT (SPECT) and stress echocardiography (Figure 1). MSCT allows us to visualise the segment of coronary that is enveloped by myocardium and to assess the haemodynamic effect that MB may have. Stress SPECT can correlate the amount of systolic luminal narrowing to the amount of ischaemia.6 A study was carried out in patients with MB in the LAD (confirmed on IVUS), in which the team sought to identify any unique findings on exercise echocardiogram in patients with symptomatic MB. They found that there was transient focal buckling at the end-systolic–early diastolic motion of the septum with apical sparing.34
Using invasive imaging, MB can be visualised on angiography (Figure 2) and on IVUS (Figures 3 and 4). With conventional angiography, a change known as the ‘milking effect’ (Supplementary Material Video 1) can be observed when there is a reduction of the minimum luminal diameter (MLD) by at least 70% during systole and there is a persistent MLD >35% by the mid-end phase of diastole. This milking effect can be further exaggerated by positive chronotropic effects (which decrease the diastolic filling time) such as those due to dobutamine infusion or if the patient is on nitroglycerin, which can cause reflex tachycardia.6,27 It is this characteristic dynamic and phasic narrowing of the affected segment that distinguishes it from a fixed plaque.1 On angiography, MB is graded according to the severity of systolic compression of LAD as grade 1, <50%; grade 2, 50–75%; and grade 3, >75%.35
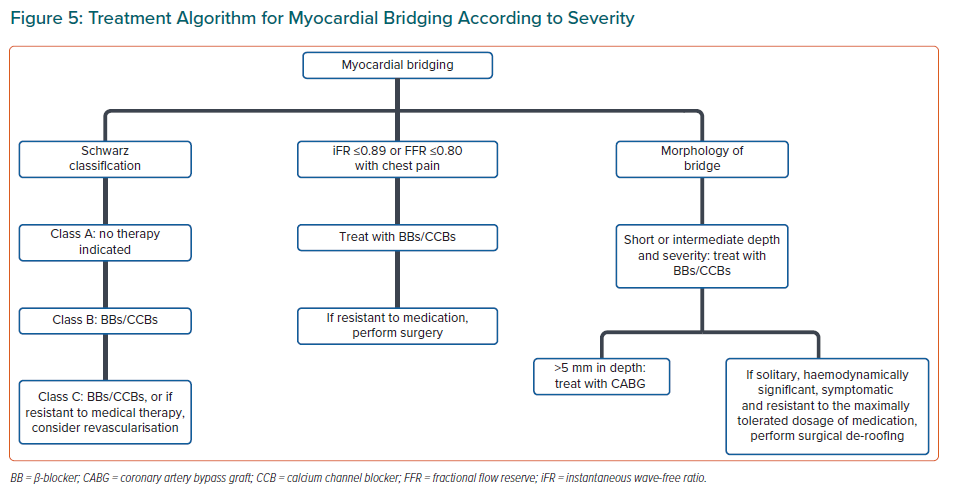
In a 5-year follow up of a retrospective study, it was found that patients with symptomatic MB who had objective signs of ischaemia (classified as type B as per Shwartz classification [Figure 5]), responded well to treatment with β-blockers or calcium channel blockers.36
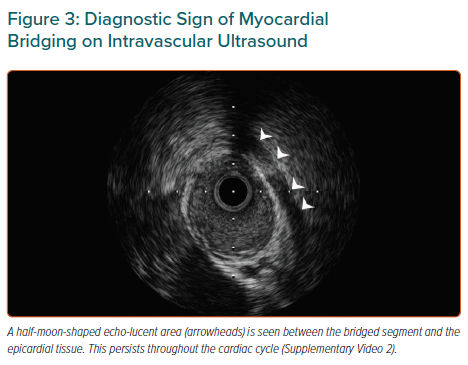
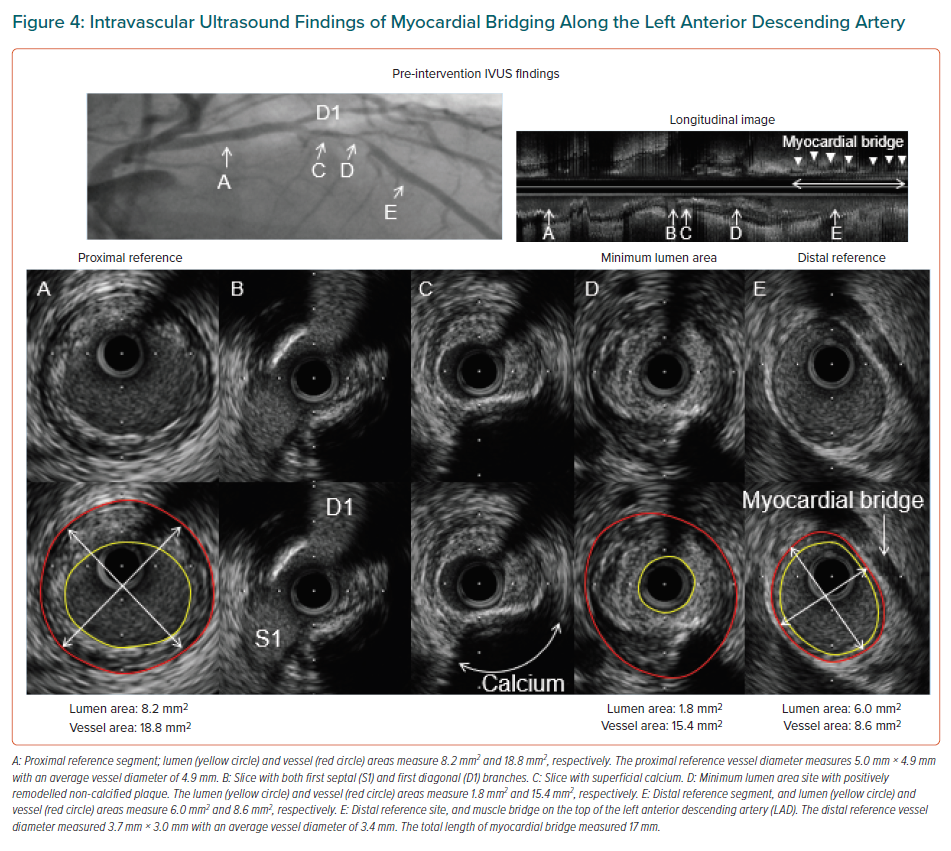
IVUS is an essential modality to diagnose MB (Supplementary Material Video 2). It is characterised by a half-moon-shaped echo-lucent area found between the bridged segment and the epicardial tissue that persists throughout the cardiac cycle (Figure 3).6
Myocardial Bridging in Hypertrophic Cardiomyopathy and Systolic Dyssynchrony
HCM is one of the causes of sudden cardiac death (SCD) due to its capricious course and heterogeneity. There is an ongoing dilemma about whether MB is associated with ischaemic mortality in HCM. The presence of wall motion abnormalities and abnormal thallium-201 scans points towards faulty perfusion associated with the MB phenomenon. Angiographic evidence of MB over LAD is found in 30–50% of HCM cases and such stenosis should be alarming in documented HCM due to possible SCD.12 Myocardial ischaemia is a well-documented cause of SCD in HCM, although it is not clear whether MB is a primary factor or a confounder in the extensive ischaemia that leads to SCD in these patients.37
According to clinical studies to date, there is no significant mortality in patients with HCM and MB as compared with HCM without MB.8 This does not necessarily rule out the role of MB in a pathological accentuation of clinical symptoms. In a study by Basso et al. in which they morphologically analysed 250 hearts, there was no systemic association between MB in HCM patients and HCM-related sudden death, although MB was commonly found in phenotypically expressed HCM patients.11 Hence, the most acceptable treatment strategy is to identify additional risk factors and to quantify the severity of MB in HCM patients.
It has long been questioned as to what percent of stenosis and length of MB are critical to affect LV functioning. Cai et al. noted a prevalence of LV systolic dyssynchrony in 25.7% (9/35) in patients with isolated symptomatic LAD MB.38 The presence of hypertension was an independent causative factor for dyssynchrony, and the combination of MB with critical stenosis and hypertension contributed significantly to systolic dyssynchrony.38 The study emphasised that stenosis >50% causes critical systolic compression and that the stenosis and length of MB have a synergistic effect on the development of dyssynchrony.38 At a critical stenosis of 50% and above, the length of the MB segment starts to have a significantly deleterious effect on LV systolic and diastolic function, which affects stroke volume. The study also emphasised that hypertension leads to diastolic dysfunction, which again contributes to dyssynchrony.38 Yetman et al. also noted that the presence of MB in HCM in children is associated with a poor prognosis.12
Myocardial Bridging Causing Arrhythmia and Role of QT Dispersion
It is believed that arrhythmias arise in patients with MB due to the limited coronary flow that occurs during periods of increased oxygen demand. Under these circumstances, the ischaemia induced by the decreased diastolic filling time leads to myocardial electrical heterogeneity.39 Increased electrical heterogeneity increases the propensity towards the development of cardiac arrhythmias. Ventricular repolarisation indices such as Tpeak–Tend interval, their dispersions and ratios, as well as QT dispersion (QTd) have all been found to be significantly increased in patients with MB, particularly during exercise.39,40
QTd is defined as the difference between the minimum and the maximum QT intervals; it is an effective marker for electrical myocardial heterogeneity and higher values are associated with an increased risk of cardiac arrhythmia.41 QTd is also a measure of the regional variability between myocardial excitability and recovery in the ECG leads used in the measurement of corrected QT interval.12 One plausible explanation for MB-associated arrhythmias is the chronic myocardial ischaemia that develops, which causes diffuse fibrosis and an increased disarray of myocardial fibres, creating an arrhythmogenic focus.12
Heart rate (HR) and HR recovery (HRR) can also be investigated in patients with MB to provide prognostic information. Under a graded exercise scheme, HRR can be used to indicate the autonomic activity of the heart, which can predict adverse cardiovascular events. This is done by subtracting the first-, second- and third-minute HR from the maximum HR achieved during exercise. In patients with MB, the HRR was blunted compared with normal patients, particularly when the LAD was involved.42 This is related to the fact that there is an imbalance in the autonomic nervous system in patients with MB.42 One may therefore propose that not only does the ischaemia-induced myocardial heterogeneity increase the propensity toward arrhythmias, but so does the increased sympathetic overactivation during exercise in patients with MB.
A study by Nishikii-Tachibana et al. found that during exercise, patients with MB developed premature ventricular contractions and non-sustained ventricular tachycardia more frequently than the general population.19 Hence, patients with MB benefit from β-blockers because they reduce systolic compression and have a negative inotropic effect.42 Although, theoretically, a multivessel bridge can cause significant ischaemia to trigger atrial dysrhythmia (fibrillation), we did not find sufficient existing research correlating MB with AF.
MI Due to Myocardial Bridging
There are two hypotheses for the incidence of MI in MB patients. One involves the dynamic compression of myocardial fibres forming the tunnel, and the other, the accelerated formation of atherosclerosis in the proximal segments of the MB. The former appears to be a prime mechanism of coronary insufficiency in psychophysical exertion in young patients, while the latter mechanism is seen in elderly patients.43
Hostiuc et al. conducted a meta-analysis and meta-regression study and found that MB increased the likelihood of major adverse cardiac events and ischaemia in the presence of hypertension, smoking and diabetes.41 Intra-coronary Doppler showed a fingertip-like anterograde blood flow, with a sharp increase in flow velocity during early diastole, followed by a steep decrease in speed, ultimately plateauing in mid-late diastole. During systole there is a decreased, absent or reversed forward flow.41 The myocardium dilates at the end of diastole, causing negative pressure in the bridge segment, which results in a ‘sucking phenomenon’.41,44 This compression is commonly eccentric rather than concentric, leading to half-moon-shaped echo-lucency as mentioned before.41,44 If the systolic compression of MB is elliptical, it is associated with 50% stenosis; and if the compression is concentric, then it translates to 75% stenosis of the luminal area.41,45
The extent and duration of compression of bridged segments of coronary arteries are of clinical importance because the compression can trigger exertional chest pain, which may mimic MI in young people. The mechanisms leading to MI are the extension of systolic compression of the bridged segment into diastole, causing diastolic lag in the filling, which reduces the coronary reserve; endothelial dysfunction in the intramural course of the artery; and haemodynamic changes proximal to a bridged segment of the artery.46
The plaque formation in the proximal artery segment could be explained by a change in the properties of the endothelium due to the chronic coronary pressure gradient that exists between the tunnelled and non-tunnelled segments, which creates a coronary pressure overload.47 Intracoronary acetylcholine normally causes vasodilation by releasing endothelium-dependent relaxing factor if the endothelium is healthy and intact. However, it causes a paradoxical vasoconstriction if atherosclerotic lesions have affected the endothelium. Bilen et al. showed that patients with MB had an increased mean platelet volume, which causes increased reactivity and induces more production of prothrombotic factors.48 A confluence of decreased prostacyclin and nitric oxide, impaired endothelin-dependent vasodilation and increased proximal atherosclerosis causes chronic ischaemic milieu and possible MI.
Coronary Artery Spasms Caused by Myocardial Bridging
Coronary spasms occur more often in MB compared with non-MB patients. Chronic endothelial and smooth muscle cell dysfunction may be involved in the genesis of spasms. Ultrastructural studies conducted by Ishii et al. showed loss of smooth muscle cells both proximally and distally to the bridge.49 Turbulent flow causes tumour necrosis factor-alpha-induced endothelial activation. The endothelial dysfunction and activation, and the turbulent flow of the blood in the tunnel can cause spasms due to ischaemia.26
Broken Heart Syndrome due to Coronary Anomalies
Takotsubo cardiomyopathy, also known as broken heart syndrome or stress-induced cardiomyopathy, is thought to be associated with the aetiology of anomalous coronary circulation. MB with a long, recurrent wraparound LAD (wrap-LAD) has been postulated to cause worsening of TCM.51 To understand this association, we need to understand coronary artery tortuosity (CAT). CAT is identified using two criteria: ≥3 consecutive curvatures ≥90°; or ≥2 consecutive turns ≥180°.50–52 Wrap-LAD was defined as any part of the vessel outreaching the apex of the LV. It is the most common anatomical anomaly associated with TCM.50 The combination of a catecholamine surge bringing a hypersympathetic response with a pre-existing coronary wrap-LAD along with MB can cause TCM.50 It was also hypothesised that plaque rupture in wrap-LAD with transient coronary artery occlusion and spontaneous thrombolysis can cause regional wall motion abnormalities in the middle and apical anterior and inferior segments, as observed by Arcari et al.50
Symptomatic MB can contribute to the worsening of TCM outcomes. Kato et al. found that TCM patients with MB had a higher in-hospital death rate than patients with TCM and no MB.20 The majority of deaths in that study, however, were from a non-cardiac origin, possibly due to the differing degree of systolic compression in each case.20
Migliore et al. suggest that MB acts as a substrate for the development of TCM. During the emotional distress that occurs at the onset of TCM, there is an increase in sympathetic drive from the surge in catecholamines that leads to an increased heart rate, aggravating the compression of the bridge, especially if LVH is present. The result is clinically symptomatic MB and the development of TCM.53
Most commonly, typical apical ballooning is found in TCM when MB is present.20 This may be explained by the fact that it is predominantly the LAD that is enveloped in myocardium.53–55
In terms of acute therapy in the setting of TCM with MB, it is logical to think that the contractility induced by the surge of catecholamines may be suppressed with the use of a negative inotrope such as a β-blocker. Kato et al. found that this may be an option.20
Coronary Artery Dissection in Myocardial Bridging
Although rare, MB is associated with dissection in some cases. The separation of intima and media requires high shear stress and severe vasoconstriction. Acetylcholine is known to cause vasoconstriction in dysregulated endothelium burdened with atherosclerosis.47 The tunnelled artery has high shear stress along with coronary spasms, which could be linked to the occurrence of coronary artery dissection. Some studies report an association of SCD with coronary artery dissection in patients with MB.56,57
Treatment of Myocardial Bridging
There is an ongoing dilemma of whether to surgically de-roof MB or perform CABG, or to treat it with medication or to place a stent. According to the literature, MB is to be treated only when the patients have symptoms.27
A treatment algorithm for MB is given in Figure 5. Medication therapy consists of β-blockers, calcium channel blockers and antiplatelet therapy. However, with refractory symptoms, surgery may be warranted. Unless there is a significant co-existing coronary vasospasm, nitrates should be avoided in patients with MB because the rebound tachycardia that they induce can exacerbate the stenosis. β-blockers decrease the HR, decrease the contractility and compression of the coronary arteries and reduce coronary filling time, thus they have been used as first-line treatment.6,33 Certain studies suggest that iFR ≤0.89 or FFR ≤0.80 with signs of chest pain and heaviness should be managed with medical or surgical intervention.31 MB that runs deep (>5 mm deep) should be treated with CABG because it has been shown to be better than surgical myotomy.58 MB that is short or intermediate in depth and severity should be treated with medication alone.58,59
Surgical de-roofing of MB poses a risk of ventricular injury leading to bleeding and the formation of aneurysm. However, in a small series of patients who have solitary, haemodynamically significant and symptomatic MB that was resistant to the maximally tolerated dosage of medication, their symptoms improved dramatically after surgical intervention.60 Percutaneous coronary intervention has been selectively used on affected vessels given that the rates of stent restenosis, fracture and stent failure are high.59,60
Atherosclerotic plaque formation in association with MB raises concern regarding a significant reduction in blood supply. It is challenging to treat such cases given that many physicians hold different opinions as to when to treat patients with significant comorbidities. Significant groups of patients have mild MB with several chest pain episodes, decreasing their quality of life, whereas other patients may have rare events with the significant deep bridging of the LAD. Such patients should always be advised to return to hospital if the pain presents for prolonged periods, with atypical manifestations of MI explained well-beforehand by cardiologists. Patient education can play an important role in the management of MB.
Conclusion
MB is a congenital condition that is commonly found on autopsy, coronary CT and angiography. It is typically a benign condition but is often under- or over-treated. MB can run a clinically dangerous course given that it has been associated with myocardial ischaemia, ACS, coronary spasm, coronary artery dissection, myocardial stunning and arrhythmia. Plaque burden slowly builds in the proximal segment and the obstructive haemodynamic effects not only affect the systole but also continue into diastole, affecting coronary perfusion. Clinical suspicion should remain high for MB in all cases of typical and atypical chest pain, especially in young patients without any coronary risk factors.
Treatment of patients with MB is based on risk factors and symptomatology. It is not yet known how MB contributes to cardiac morbidity and prognostic outcomes in cardiac patients. The treatment depends on the depth of MB and on the severity of haemodynamic disturbance compromising cardiac function. Currently, medication is the accepted first-line therapy, while surgical de-roofing and CABG are reserved for severe stenosis.