The burden of residual cardiovascular risk remains an important concern for patients with coronary artery disease (CAD) despite major advances in secondary cardiovascular prevention having been achieved over the past decades.1 Further reduction in low-density lipoprotein cholesterol by treatment with statins and more recently with proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors, or reducing triglyceride levels using high-dose icosapent ethyl are associated with better clinical outcomes, underscoring the importance of the lipid component of this residual risk.2–5 Nevertheless, recent randomised controlled trials (RCTs) have demonstrated that selectively targeting inflammation further improves clinical outcomes in patients with atherosclerosis, indicating that inflammation plays an important role in the burden of cardiovascular risk.6 Indeed, the use of canakinumab, which inhibits interleukin (IL)-1β without lowering cholesterol or blood pressure, was associated with a significant reduction in major adverse cardiovascular events (MACE) in the CANTOS study, with the greatest clinical benefit being observed among patients with the greatest reductions in IL-6 and C-reactive protein.7
Colchicine is an old drug with several anti-inflammatory properties, preventing microtubule polymerisation at low dose and promoting microtubule depolymerisation at high dose, acting on neutrophils and endothelial cells and inhibiting the nucleotide-binding and oligomerisation domain (NOD)-like receptor protein 3 (NLRP3) inflammasome, thus blocking the downstream upregulation of pro-inflammatory IL 1β and IL 18.8–12
Recent RCTs and meta-analyses have shown that colchicine may reduce MACE in patients with CAD, supporting the inflammation hypothesis of atherosclerosis.6,13–15 Nevertheless, uncertainty exists about the effects of colchicine on mortality outcomes. In addition, absolute treatment effects of colchicine on clinical outcomes are yet to be reported.15,16 We systematically reviewed evidence from trials to determine the association between colchicine treatment and specific cardiovascular outcomes, adverse events and mortality outcomes.
Methods
We performed a systematic review and meta-analysis of RCTs examining the association between add-on colchicine treatment and clinical outcomes in patients with CAD. The study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.17 The study protocol was registered on PROSPERO (the international prospective register of systematic reviews), and the number CRD42021248874 was assigned.
Search Strategy
We searched Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library), ClinicalTrials.gov databases and the main international conference proceedings for all RCTs assessing the effects of colchicine treatment on clinical outcomes in patients with CAD. Searches were undertaken independently by two reviewers (FC and MS) from inception of the databases until 1 March 2021. No language, publication date or publication status restrictions were applied. We used the following MeSH terms: “colchicine” and “coronary artery disease” or “CAD” or “chronic ischaemic heart disease” or “atherosclerosis” or “angina” or “acute coronary syndrome” or “myocardial infarction” or “percutaneous coronary intervention” or “PCI” or “angioplasty” or “drug eluting stent” or “DES” or “bare metal stent” or BMS”.
Selection of Studies and Inclusion and Exclusion Criteria
Study eligibility was independently assessed by two reviewers (FC, MS) on the basis of titles, abstracts and full-text reports. Discrepancies in the study selection were discussed and resolved with another reviewer (GF). Eligible studies included participants with CAD and compared individuals receiving add-on colchicine with those receiving placebo or no treatment; and provided clinical outcome data at follow-up. We excluded studies without a randomised design as they are prone to bias from confounding by indication and studies including participants not affected by CAD or specific groups of patients requiring colchicine treatment, such as those affected by familial Mediterranean fever or gout.
Outcome Measures
The primary efficacy outcome was the composite of MACE according to the definition used in each study, which usually comprised a combination of either all-cause or cardiovascular mortality, MI, stroke, with or without coronary revascularisation. Nevertheless, we excluded all-cause mortality from the definition of MACE and included cardiovascular mortality to reduce heterogeneity. The primary safety outcome was gastrointestinal adverse events. Secondary clinical endpoints were MI, stroke and any coronary revascularisation. With respect to mortality outcomes, we assessed all-cause death, cardiovascular death and non-cardiovascular death. The latter was obtained directly from the study reports or was calculated as the difference between all-cause deaths and cardiovascular deaths when it was not directly reported in the study.
Data Extraction and Quality Assessment
Two reviewers (FC and MS) independently extracted data from eligible studies using a standardised data abstraction form and independently entered outcome data into a Microsoft Excel spreadsheet (2016 version). Another reviewer then manually cross-checked these, referring to the original source data when discrepancies were identified. Any disagreements of collected information between the two reviewers were reconciled through discussion with a third reviewer (GF).
Data were extracted on populations studied, colchicine dose, length of follow-up, outcome definitions, inclusion and exclusion criteria, sample size, number of patients experiencing adverse events, mortality outcomes and follow-up time.
Two independent reviewers (FC, MS) evaluated the methodological quality of the included studies using the revised Cochrane risk-of-bias tool (RoB 2.0) assessing five domains of bias for each outcome:18
- Randomisation process;
- Deviation from intended interventions;
- Missing outcome data;
- Measurement of the outcome; and
- Selection of the reported results.
Any disagreement was resolved with a third reviewer (GF).
Data Synthesis and Analyses
Outcome data were treated as incidence rate which is based on counts of events over time per person-year recorded separately for each study arm, for example colchicine group and control group, owing to the heterogeneity of follow-up duration among studies. We used the pooled incidence rate ratio (IRR) with 95% confidence intervals (CI) to measure the effect size. A mixed-effects Poisson regression model with random intervention effects at the study level was used to estimate the pooled IRR as this method is without bias and held with a large number of zeros in the data as well as when there is high heterogeneity.19–21 Risk differences with their 95% CI for each endpoint between the colchicine arm and the control group were estimated by the Poisson model using the delta method.
The number of patients needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful (NNTH) outcome with a 95% CI were calculated from risk differences and defined the inverse of risk difference.22,23 The presence of heterogeneity among studies was evaluated with the Cochran Q test with p≤0.1 considered of statistical significance, estimating the between-study variance tau square. The proportion of variability in effect estimates due to between-study heterogeneity was summarised using the I2 test to evaluate inconsistency. I2 values of 25%, 50% and 75% have been assigned adjectives of low, moderate and high heterogeneity, respectively.24
The presence of publication bias for each endpoint was investigated by visual estimation with the use of contour-enhanced funnel plots.25 To address potential sources of heterogeneity, we performed a pre-specified subgroup analysis according to colchicine dose, <1 mg /daily or ≥1 mg/daily, with respect to main clinical outcomes including MACE, MI, stroke, all-cause death and gastrointestinal adverse events. Another pre-specified subgroup analysis based on the type of clinical presentation, i.e. acute coronary syndrome (ACS) versus chronic coronary syndrome (CCS), was performed although this analysis was restricted to a limited number of studies which reported data split according to clinical syndrome.
A formal interaction test between treatment effects of each subgroup was done as previously recommended.26 No other subgroup analyses were undertaken by patient level characteristics owing to the risk of ecological bias. A leave-one-out sensitivity analysis was performed by leaving out exactly one study to assess the consistency of the results. The statistical level of significance was two-tailed p<0.05 for treatment effects and p<0.1 for the interaction test. All analyses were undertaken using R 3.6.3 and Stata/MP version 16 (StataCorp LP) software.
Results
Study Selection and Characteristics
The search strategy and selection process are summarised in Supplementary Material Figure 1. A total of 840 unique articles were identified from the literature searches. After screening of the title, abstract and full text, 10 articles originating from 10 RCTs were eligible for inclusion.13,14,16,27–32 Cohort characteristics are summarised in Table 1 and Supplementary Material Table 1, extracting data for the present analyses from the primary trial reports.
A total of 12,819 participants were included in the primary analyses from 10 unique RCTs. Eight studies compared add-on colchicine with placebo and two studies compared add-on colchicine with standard treatment.13,14,16,27,28–32 The mean (SD) age of trial participants ranged from 57.2 (11.7) years to 67 (9.2) and 10,680 (83.3%) participants were men.28,29 The sample size of the individual studies ranged from 44 to 5,522. The overall prevalence of diabetes ranged from 12 to 100%.27,30 Across the 10 trials, median follow-up was 6 months (interquartile range 1–23, minimum to maximum 1–28.6 months). Treatment duration exactly matched follow-up duration in all included studies except the Colchicine-PCI study where treatment duration was limited to 1 day while follow-up was 30 days, and the COPS study where it was 1 year, and follow-up duration was 400 days.16,32
Quality Assessment
Supplementary Material Figure 2 presents the risk-of-bias assessment for individual trials. Overall, six (60%) studies were judged to have ‘some concerns’, and one (10%) to have ‘high risk’ of bias. The main reasons for some concerns were bias in selection of the reported results (6; 60%) and bias due to missing outcome data (6; 60%). Treatment effects of two (20%) of the studies could be contaminated owing to the open label design. Contour-enhanced funnel plots showed the presence of significant asymmetry for non-mortality endpoints, potentially due to publication bias and other issues, whereas mild asymmetry was found for mortality endpoints (Supplementary Material Figures 3–10).
Study Heterogeneity
Significant heterogeneity was observed for the primary safety outcome of gastrointestinal adverse events, as well as for stroke, and non-cardiovascular death (Supplementary Material Table 2). Moderate inconsistency was found for the primary endpoint of MACE, coronary revascularisation and mild inconsistency was detected for the remaining outcome measures (Supplementary Material Table 2).
Primary Outcomes
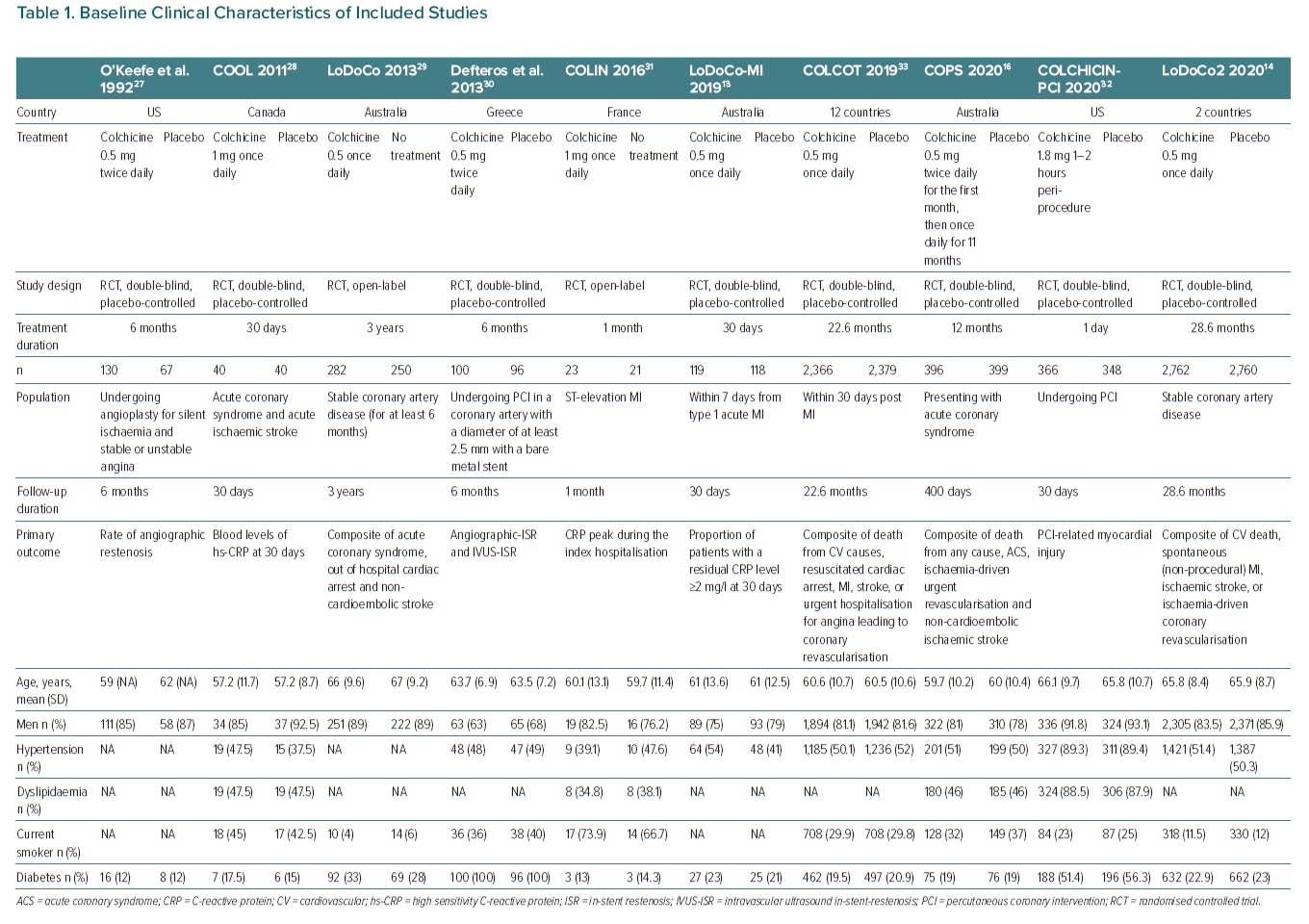
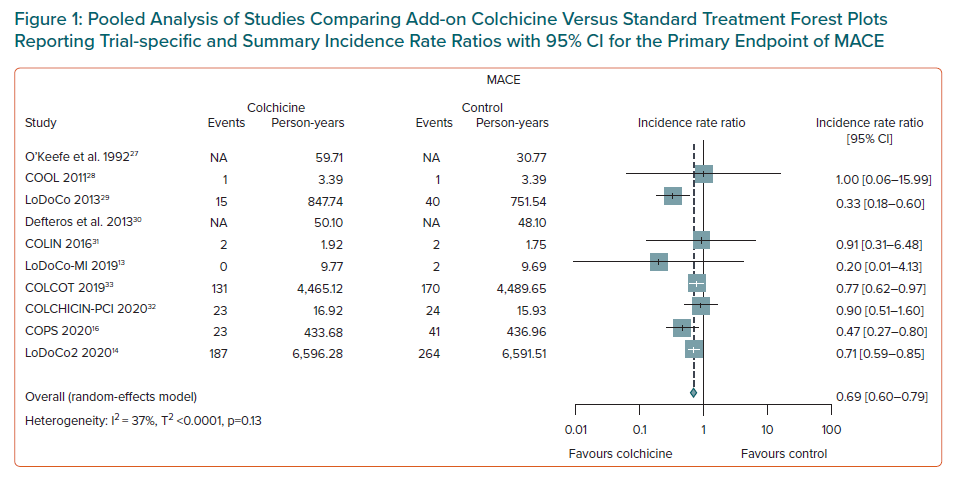
Associations between colchicine use and the relative and absolute risks for outcomes are presented in Supplementary Material Tables 3 and 4. Overall, there was strong evidence that, add-on colchicine therapy reduced the risk of MACE compared to standard treatment (IRR 0.69; 95% CI [0.60–0.79]; NNTB = 28, number of events avoided = 35 per 1,000 patients treated, n=8 studies, Figure 1) and significantly increased the risk of gastrointestinal adverse events (IRR 1.69; 95% CI [1.12–2.54]; NNTH = 10, number of events caused = 99 per 1,000 patients treated, n=8 studies, Figure 2).
Secondary Outcomes
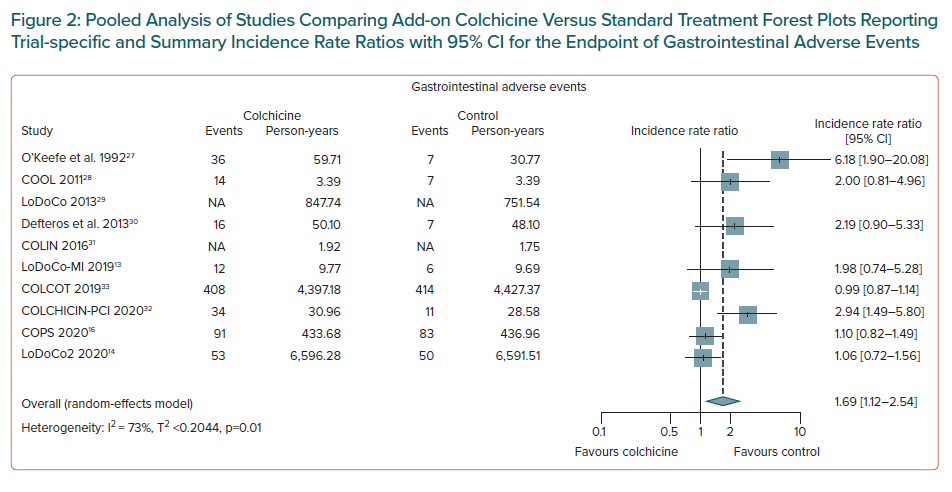
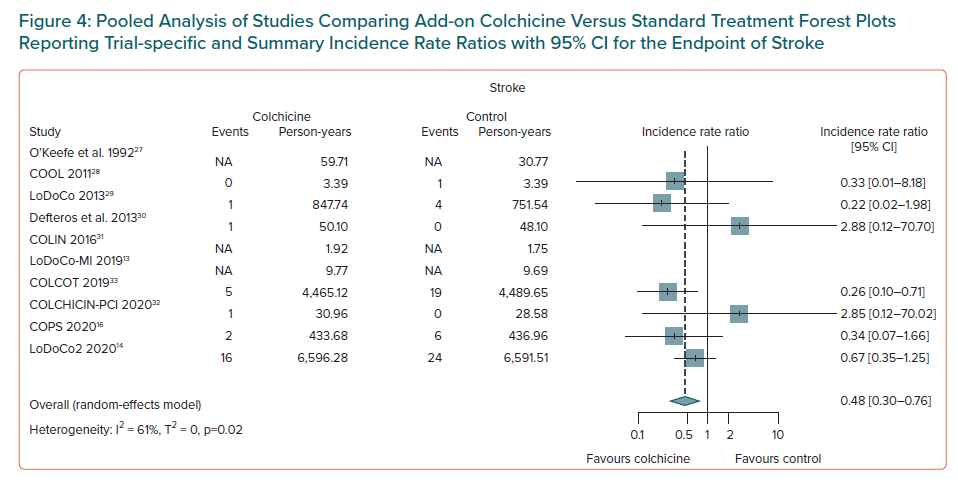
Colchicine use was associated with a lower risk of MI (IRR 0.77; 95% CI [0.64–0.93]; NNTB = 95, number of events avoided = 10 per 1,000 patients treated; n = 8 studies, Figure 3), and of ischaemic stroke (IRR 0.48; 95% CI [0.30–0.76]; NNTB = 155, number of events avoided = 6 per 1,000 patients treated, n=7 studies, Figure 4). Weak evidence was found for an association between colchicine and a lower risk of coronary revascularisation (IRR 0.66; 95% CI [0.41–1.05]; NNTB = 51, number of events avoided 20 per 1,000 patients treated, n=4 studies, Supplementary Material Figure 11).
Mortality Outcomes
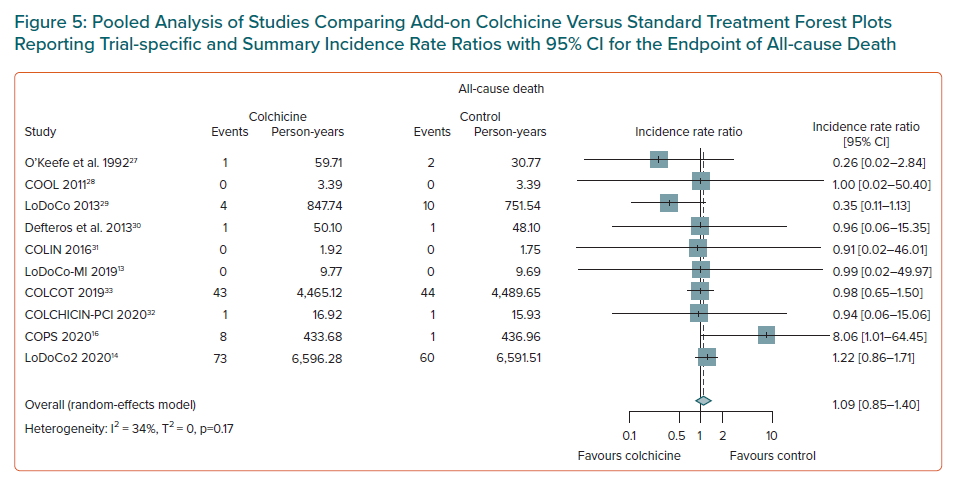
There was no evidence of an association between colchicine and an increased risk of all-cause death (IRR 1.09; 95% CI [0.85–1.40], n=10 studies, Figure 5), or of cardiovascular death (IRR 0.75; 95% CI [0.51–1.12], n=9 studies, Supplementary Material Figure 12). Weak evidence of an association of colchicine with a higher risk of non-cardiovascular death was found (IRR 1.45; 95% CI [1.04–2.02], NNTH 396, number of events caused 3 per 1,000 patients treated, n=9 studies, Supplementary Material Figure 13).
Subgroup Analyses
We conducted two pre-specified analyses on colchicine dose and clinical syndrome – ACS versus CCS. With respect to the analysis of colchicine dose, we found a significant interaction with the risk of gastrointestinal adverse events (IRR 1.03; 95% CI [0.91–1.15]) in patients receiving low-dose colchicine and IRR 2.91; 95% CI [1.91–4.44] in patients receiving high-dose colchicine; p<0.0001 for interaction (Supplementary Material Table 5 and Figure 14). Of note I2 and the between-study heterogeneity was zero in stratified analyses of colchicine dose for gastrointestinal adverse events and I2 largely decreased for the endpoint of stroke (Supplementary Material Table 6). By contrast, no evidence for a modification of treatment effect of colchicine dose for the endpoints of MACE, MI, stroke and all-cause death (Supplementary Material Table 5 and Figures 15–18) was observed.
With respect to the analysis of the type of clinical syndrome, we found little evidence for an interaction with the risk of MI (IRR 0.91; 95% CI [0.71–1.18]) in patients presenting with ACS and IRR 0.65; 95% CI [0.50–0.84] in patients presenting with CCS, p=0.07 for interaction (Supplementary Material Table 6 and Figure 20). Supplementary Material Table 8 reports the heterogeneity measures of this subgroup analysis. No evidence for a modification of treatment effect for the type of clinical syndrome was observed for MACE, stroke and all-cause death (Supplementary Material Table 7 and Figures 19, 21 and 22)
Sensitivity Analyses
The results of the leave-one-out sensitivity analyses for each outcome obtained by leaving out exactly one study are presented in Supplementary Table 9. Overall results were consistent with the main analysis for the primary endpoint of MACE, all-cause death and cardiovascular death. Nevertheless, the following small variations in treatment effects were observed: the omission of the LodoCo2 study slightly attenuated the treatment effect on the risk of MI and non-cardiovascular death, the omission of COLCOT slightly attenuated the treatment effect on stroke and increased the beneficial effect of colchicine on revascularisation, finally the omission of one study attenuated the harm for gastrointestinal adverse events.13,14,27
Discussion
The present study is a comprehensive meta-analysis including clinical outcomes data from 10 RCTs and more than 12,800 patients with CAD showing the benefit of an add-on colchicine to standard treatment in reducing the risk of MACE, MI, stroke and to a lower extent any coronary revascularisation at follow-up. These data also found an association between colchicine and gastrointestinal adverse events and showed that this association is dependent on colchicine dose, with low-dose colchicine (<1 mg daily) having a better safety profile and high-dose colchicine (≥1 mg daily) carrying an increased risk of adverse events. Of note, the protective effects on cardiovascular outcomes were not affected by colchicine dose, while the benefit of colchicine on the risk of MI was attenuated in patients presenting with ACS, compared to patients presenting with CCS. The study data show no evidence of an association between colchicine and mortality either from any cause or cardiovascular causes and provide weak evidence for an association of colchicine with an increased risk of non-cardiovascular death as indicated by a wide CI.
These data will inform shared decision-making around initiation and optimal dosage of colchicine treatment for secondary cardiovascular prevention, especially in patients with a high absolute risk of future cardiovascular outcomes. Such discussions will become increasingly important among patients for whom traditional treatment strategies for the reduction of cardiovascular residual risk may not be implemented, such as patients at high risk of bleeding who cannot tolerate a prolonged dual antiplatelet therapy or dual antithrombotic strategy, or patients who are unable to receive aggressive LDL cholesterol reduction therapy owing to intolerance to high-dose or any statin treatment and/or may not undergo PCSK-9 inhibitor treatment owing to prohibitive treatment costs.
Compared with previous meta-analyses of clinical outcomes of colchicine use in this setting, one important difference in the present analysis includes the use of incidence rate and IRR for all eligible trials and the estimation of absolute treatment effect measures.15,33–35 By contrast, previous meta-analyses limited extraction to analyses reporting relative risks or HRs which do not account for heterogeneity of follow-up duration and may introduce bias. Analyses of absolute treatment effects show that the benefits of colchicine on MACE are of clinical relevance given the NNTB = 28 corresponding to 35 events avoided per 1,000 patients treated. While the highest relative risk reduction associated with colchicine was observed for stroke (IRR 0.48), a higher absolute risk reduction was found for MI (NNTB = 95) and coronary revascularisation (NNTB = 51).
Our pre-specified subgroup analysis showed an interaction between colchicine and the type of clinical presentation with the risk of MI, indicating the highest benefit among patients with CCS. This finding is in agreement with a previous meta-analysis showing a higher benefit of colchicine on cardiovascular outcomes in patients with CCS compared with patients with ACS.34 Nevertheless, the included studies in the ACS group were heterogeneous with respect to timing of colchicine administration. A previous post-hoc analysis of the COLCOT study suggested that an earlier onset of colchicine administration could be associated with better clinical outcomes among patients with ACS with the greatest benefit observed when colchicine was started within 3 days from MI, during the highest inflammatory window.36
Our data also provide important evidence with respect to the effects of colchicine on mortality outcomes. COPS reported a signal towards higher total mortality in the colchicine group that was driven by a higher rate of non-cardiovascular death.16 Analyses of causes of death revealed that non-cardiovascular death was related to sepsis in four out of five cases, however three out of four patients with sepsis-related deaths in the colchicine group discontinued study medication early in the trial (within the first 30 days) and were not taking colchicine at the time of death.16 In LoDoCo2, the incidence rates of death from any cause and non-cardiovascular death were higher with colchicine than with placebo.14 The observed between-group difference in the incidence of non-cardiovascular death was not significant, as shown by the 95% CI, although the HR of 1.51 was deemed of potential concern.
We found no evidence that colchicine could affect the risk of all-cause death or cardiovascular death and provided weak evidence for an association of colchicine with an increased risk of non-cardiovascular death. In interpreting the latter finding, one should consider that the definition of non-cardiovascular death could be subject to bias and misclassification as there was no systematic distinction between unknown or missing causes of death and all other causes of death in the included studies. Also, we calculated non-cardiovascular deaths as the difference between all-cause deaths and cardiovascular deaths when not specifically reported in each study. Further, the mixed-effects Poisson regression model with random intervention effects that we used in these analyses could not be extended to handle the situation of competing risks, such as between cardiovascular and non-cardiovascular deaths given the lack of data on individual participants, therefore we cannot rule out that a small non-significant advantage in terms of cardiovascular death associated with colchicine use could translate into an apparently higher, yet overestimated, risk of non-cardiovascular mortality. Also, the absolute treatment effect of colchicine on non-cardiovascular death was small with an NNTH of 396, corresponding to less than three events caused per 1,000 patients treated. Finally, we cannot rule out the role of chance underlying a potential association between colchicine and non-cardiovascular death at follow-up, therefore ongoing studies, such as CONVINCE (NCT02898610) and CLEAR SYNERGY (NCT03048825), will provide important evidence about this issue.
Limitations
We observed statistically significant heterogeneity across studies for several outcomes, therefore caution should be used when interpreting the results. For the outcomes of stroke and gastrointestinal adverse events, the observed heterogeneity was largely explained by pooling according to two different colchicine dosages – low-dose or high-dose. For other outcomes, the observed heterogeneity could not be explained by differences in colchicine dose or the type of clinical presentation – ACS or CCS. However, other differences in populations of interest and study designs could have contributed to the observed variation. Evidence was found of publication bias for certain outcomes confirming the findings of previous studies that showed adverse events are more likely to be reported in RCTs when they are statistically significant. We also acknowledge the lack of individual patient-level data which does not allow us to assess the effect of baseline patient characteristics on treatment effects and limits the ability to identify population subgroups both at potentially elevated risk and at potentially greater drug-derived benefit.
Across all included trials, the definition of the primary endpoint and of other outcomes varied. For instance, several studies referred to MACE as an outcome including coronary revascularisation in addition to all-cause death, MI and stroke, but in other studies MACE did not comprise coronary revascularisation. Another limitation includes the notion that RCTs often select populations with less frailty and multimorbidity which may not be representative of the real world. Further, only 16.7% of participants included in the meta-analysis were women and therefore our findings cannot be directly applied to this patient population. Finally, the median follow-up duration was 6 months and additional studies with longer follow-up are needed to confirm the long-term efficacy of colchicine, and the time points at which clinical outcomes occurred varied across studies, yet the use of a mixed-effects Poisson regression model with random intervention effects is appropriate when dealing with different follow-up durations.
Conclusion
This study found strong evidence to suggest that colchicine treatment is associated with a lower risk of MACE, including MI and stroke in patients with CAD, in particular among those with CCS, and found no evidence of increased risk of all-cause or cardiovascular death.
Colchicine is associated with a higher risk of gastrointestinal adverse events that can be prevented by using a low-dose regimen. The evidence for an association of colchicine with non-cardiovascular death is weak and the absolute treatment effect is small. Nevertheless, adequately powered larger studies with longer follow-up are needed to dispel any doubts about any potential harm of colchicine with respect to non-cardiovascular death.
The relative and absolute beneficial treatment effects of colchicine treatment on cardiovascular outcomes outweigh the potential harm of non-cardiovascular death, which is of debatable clinical relevance, thus supporting colchicine use for cardiovascular risk reduction in secondary prevention.
Click here to view Supplementary Material.
Clinical Perspective
- Among patients with coronary artery disease, add-on colchicine therapy compared to standard treatment is associated with a significant reduction of the risk of major adverse cardiovascular events by 31% of MI by 23% and stroke by 52%.
- There is no evidence that colchicine is associated with an increased risk of all-cause death or cardiovascular death and there is weak evidence of an association of colchicine with a higher risk of non-cardiovascular death.
- Colchicine is associated with a higher risk of gastrointestinal adverse effects that can be prevented by using a low-dose regimen (<1 mg daily).
- The absolute beneficial treatment effect of colchicine treatment on cardiovascular outcomes largely outweighs the absolute harmful treatment effect on non-cardiovascular death, which is of debatable clinical relevance, thus supporting colchicine use for cardiovascular risk reduction in secondary prevention.