Cardiovascular disease (CVD) remains the leading cause of death worldwide with the weight of cardiovascular (CV) morbidity and mortality borne inequitably by people with adverse social determinants of health.1,2 The COVID-19 pandemic has further magnified these disparities in the US, where black, hispanic and Asian populations experienced a disproportionately high number of CV deaths.3 Social determinants of health (SDOH) and structural racism influence access to quality healthcare, including advanced therapies, such as transcatheter edge-to-edge repair (TEER) of the mitral valve, transcatheter mitral valve replacement (TMVR), transcatheter aortic valve replacement (TAVR) and transcatheter left atrial appendage occlusion (LAAO), which subsequently affects CV morbidity and mortality.4 In this article we review the impact of socioeconomic position (SEP), one of the SDOH components, on usage and access to resources in coronary and structural interventions.
Socioeconomic Position and its Impact on Cardiovascular Disease Outcomes
The World Health Organization defines SDOH as ‘the circumstances in which people are born, grow, live, work, and age, and the systems put in place to deal with illness’.5 SDOH are highly interrelated and encompass SEP, social support, culture, access to medical care and residential environments. The four key areas that influence SEP are the level of education attained, employment status, income level and sociodemographics of an individual’s neighbourhood (Figure 1).4 Structural racism is a fundamental driver of health disparities and has historically influenced SEP and therefore access to healthcare.6
In the US, a higher prevalence of traditional risk factors (smoking, hypertension, unhealthy lifestyle – including a lack of sufficient physical activity and maintenance of a heart healthy diet) is encountered among people living in lower SEPs.2,7 However, CVD mortality is better explained by an individual’s educational attainment, household income, residential environment and access to healthcare than by traditional CV risk factors.8,9 The increased CVD burden borne by patients from low SEPs is influenced by psychosocial, behavioural and biological factors.2 It has also been shown that there is an inverse correlation of the median income of a neighbourhood and cardiovascular-related mortality.10 An individual’s environment can also have a significant impact on their cardiovascular health and outcomes. These SDOH include access to healthy foods, access to safe areas to live and to be physically active and also includes access to quality healthcare.2 Reduced access to these key determinants of health can negatively affect CV outcomes leading to frequent readmissions for heart failure, disparities in acute MI (AMI) management, implantable cardioverter defibrillator placement, cardiac rehabilitation, prescriptions, among other things.11–14
Intersection Between Sex, Ethnicity and Socioeconomic Position
There is an overrepresentation of women, black, hispanic, native American or native Alaskan people among communities living in lower SEPs.2 Our review will include evidence on sex and racial disparities seen in the usage and access to resources in coronary and structural interventions.
Socioeconomic Disparities in Usage and Resource Access in Coronary Revascularisation Procedures
SEP may affect the receipt of percutaneous coronary intervention (PCI) as well as its outcomes. While many studies have focused on sex, race and insurance status as surrogates of SEP, increasingly there have been greater efforts to characterise SEP and deprivation by including neighbourhood-level indicators of SEP such as median household income (MHI), employment status, education level and housing.15–17 A recent systematic review of 181 studies derived from mainly high-income countries (81%) showed an association between lower SEP – education, income, occupation, insurance, or a composite – and receipt of reperfusion although study findings were inconsistent and the study did not separate the means by which reperfusion was achieved (thrombolysis versus PCI). An analysis of 6.6 million admissions with AMI between 2004–2014 reported that patients in the lowest quartile MHI were significantly less likely to receive coronary angiography or PCI, with the greatest effect seen in patients presenting with ST-elevation MI (STEMI).18 Residents in low-income areas were less likely to receive catheterisation within 24 hours of a STEMI or within 48 hours of a non-STEMI (NSTEMI) than patients from high-income areas.2 The authors speculated that the difference may be due to the perception that individuals with low income would be less likely to afford post-PCI antiplatelet agents.19 There is a strong correlation between education and health literacy.20,21 Individuals with poor health literacy are more likely to be non-compliant with their medications.22
Even when patients with low SEP presenting with AMI do receive invasive therapy, they are more likely to experience longer reperfusion times and less likely to receive a drug-eluting stent during PCI or guideline-directed medical therapy at follow-up.19,23,24 Similarly, in an analysis of 4,380,827 admissions for NSTEMI in the US, patients in the lowest MHI quartile were more likely to be managed medically and less likely to receive coronary angiography.25 Disparities in the receipt of revascularisation are not limited to just AMI, analysis of 3,906 out-of-hospital cardiac arrest (OHCA) patients admitted alive and captured in the Swedish Registry for Cardiopulmonary Resuscitation, receipt of early coronary angiography was associated with increasing income (OR 1.31; 95% CI [1.01–1.68]) and (OR 1.67; 95% CI [1.29–2.16]) for the two highest income quartiles compared to the lowest income quartile.26
Other studies have used the area deprivation index (ADI) to define patients’ SEP at the neighbourhood level to study the relationship between SEP and clinical outcomes in patients undergoing PCI. Analysis of 146,939 patients in New York State’s PCI Reporting System found that patients in the highest ADI quintile (most deprived) were most likely to be young, female or black and live in rural parts of the country with the highest prevalence of comorbidities, such as diabetes, congestive cardiac failure and chronic lung disease.17 Patients in the highest ADI quintile had the highest odds of 30-day mortality (OR 1.24, 95% CI [1.03–1.49]) with the greatest odds for 30-day readmissions (OR 1.17; 95% CI [1.04–1.32]) even after adjustment for differences in baseline covariates and race.17 Similarly in an analysis of the national readmissions database of 833,344 patients undergoing PCI in the US, the lowest quartile MHI was independently associated with a 5–8% increased risk of unplanned 30-day readmissions, with similar findings reported for cardiac and non-cardiac causes for readmission.27
In an analysis of 13,770 patients undergoing PCI in the UK, patients with the highest English Index of Multiple Deprivation (IMD) score were younger, with more comorbidities and were least likely to undergo PCI for elective indications.28 No significant differences in major adverse cardiovascular events (MACE) or complications were reported among the quintiles of SEP, but long-term mortality increased progressively across IMD quintiles, with those patients in the most deprived quintile (Q5) having the greatest age-adjusted HR for death compared with those patients that were least deprived (Q1) (HR 1.65; 95% CI [1.40–1.94]).28 SEP may also be associated with the symptomatic benefit following PCI, for example, a two-centre study of 1,346 consecutive patients undergoing PCI reported that health-related quality of life scores were significantly lower at baseline (difference 0.08; 95% CI [0.01–0.14]; p=0.003) and at follow-up (difference 0.12; 95% CI [0.07–0.17]; p<0.001) for patients with low SEP.29 Furthermore, low SEP was associated with a lower mean improvement in self-reported health status after PCI.29
SEP may also affect outcomes following cardiac surgery. An analysis of 240,221 records from 31 hospitals in the UK reported that the highest quintile of IMD was associated with an increased risk of in-hospital mortality for both isolated coronary artery bypass surgery as well as all cardiac surgical procedures, even after adjustment for operative risk as calculated with logistic EuroSCORE.30 Similar findings were reported for longer-term follow-up at 10 years.30 Following adjustment for logistic EuroSCORE, BMI and smoking history, patients belonging to the most deprived group undergoing all-cardiac surgery had a significantly increased risk of reduced survival relative to the patients belonging to the least deprived group, with significant HRs observed for patients undergoing isolated coronary artery bypass grafting (CABG), isolated valve surgery, and combined valve and CABG surgery.30 Other measures, such as the Distressed Communities Index (DCI), a composite ranking by postal code that accounts for seven component metrics that encompass unemployment, education level, poverty rate, median income, business establishments, job growth and housing vacancies has been used to assess SEP and outcomes following cardiac surgery. An analysis of all patients undergoing isolated CABG between 2010 and 2017 (n=19,756) in the Virginia Cardiac Services Quality Initiative database, reported that higher DCI scores (lower SEP) was associated with increased in-hospital mortality, with DCI independently associated with operative mortality after CABG (OR 1.14; 95% CI [1.04–1.26]) per 25-point increase in the DCI.15 Similarly, other studies have shown patients with a lower individual income level had an increased risk of poorer long-term outcomes after CABG as compared with the highest individual income level quartile.31 Finally, while ADI has been shown to be associated with in-hospital and 30-day mortality following cardiac surgery in New York, it was not associated with 30-day readmissions.32
Given the significant changes in racial, ethnic and sex composition by age in the US (50% of children are non-hispanic white whereas 80% of elderly are white and there are more boys yet more elderly women) it is difficult to determine sex and racial disparities in rates of diagnosis, especially for diseases that vary with age.33 Numerous publications report disparities in usage of CV procedures between men and women. In a meta-analysis of >700,000 patients with STEMI, women received less antiplatelet therapy, primary PCI and experienced significantly longer delays to first medical contact (mean difference 42.5 minutes) and door-to-balloon time (mean difference 4.9 minutes).34 The HERMES study used machine learning to demonstrate that symptoms of AMI are no different between women and men, however interpretation of these symptoms may affect treatment.35 Delays can be due to a lack of patient or physician recognition and/or physician biases given the known lower likelihood of obstructive coronary artery disease (CAD) or higher procedural complications in women.36 Moreover, studies have shown that depression, caregiving demands and poorer SEP all influence the use of recommended medical care by women.37,38 Black patients in the US, especially black women, undergo fewer cardiovascular procedures and potential reasons include long-term consequences of systemic racism such as lack of trust in the healthcare system, provider biases and low SEP.4
Although lack of insurance plays a role in disparities of care, providing insurance coverage did little to reduce disparities in coronary revascularisation in a study based in Massachusetts.39 Furthermore, providing a co-payment voucher for P2Y12 inhibitor increased patient-reported medication adherence but did not lead to a significant reduction in 1-year MACE.40 Thus, factors that affect SEP and the built environment, including knowledge, attitudes, complexity of prescribed regimens, difficulties accessing medications, such as poor transportation, play a role in the underuse of cardiovascular therapies.
Socioeconomic Disparities in Usage and Resource Access in Percutaneous Valvular Interventions
Socioeconomic Factors and Transcatheter Aortic Valve Replacement
A national cross-sectional analysis of Medicare claims data demonstrated that for each US$1,000 decrease in MHI, the number of TAVR procedures performed per 100,000 Medicare beneficiaries was 0.2% (95% CI [0.1–0.4]) lower.41 Furthermore, for each one-unit increase in the DCI score, the number of TAVR procedures performed per 100,000 Medicare beneficiaries was 0.4% (95% CI [0.2–0.5]) lower (p<0.001).41 Interestingly, rates of TAVR in this study were lower in postal code areas with higher proportions of black and hispanic patients, despite adjusting for differences in SEP, clinical comorbidities, and age.41 Other studies report that with every US$10,000 increase in income, the odds of receiving TAVR increased by 10% (p=0.05), while non-black patients were twice as likely to receive TAVR than black patients (OR 2.81 95% CI [1.01–7.85]; p=0.048).42 Genetic differences among black and hispanic patients may account for lower rates of aortic valve stenosis on echocardiography.43,44
These disparities may not only reflect systemic racism and differences in referral patterns, but also inequitable introduction of such new technologies at the hospital or healthcare system level that may generate or widen existing disparities. Nathan et al. studied characteristics of 583 hospitals that developed TAVR programmes in the US between 2012–2018 using Medicare data, along with patients’ SEP that these hospitals served to examine whether there were disparities in the diffusion of newer technologies by SEP.45 Hospitals that started TAVR programs during the initial growth of TAVR in the US had higher MHI and were less likely to be based in areas with lower DCI scores.45 During this initial growth of TAVR programmes, hospitals serving wealthier patients were more likely to start programmes leading to disparities in the dispersion and access to TAVR, with lower rates in poorer communities.45 Data from the New York State registry suggests that the initial SEP-based disparities in access to the procedure resolved over time, although it is unclear whether this is a reflection of what is happening nationally, particularly in rural areas where SEP differs the most.46
Socioeconomic disparities may not only be relevant with regards to access to TAVR but may also be associated with poorer outcomes in patients who receive TAVRs. A retrospective analysis of patients undergoing TAVR at the Kaiser Permanente Los Angeles Medical Center demonstrated that a high ADI was independently associated with longer term mortality (HR 1.86; 95% CI [1.33–2.59]), although was not associated with increased risk of hospitalisations for heart failure.47
Socioeconomic Factors in Mitral Valve Interventions
Transcatheter Mitral Valve Replacement
SEP influences choice of technique and clinical outcomes after mitral valve surgery. In a report from the Society of Thoracic Surgeons, patients with a lower SEP had more urgent, non-degenerative MV pathology, received less surgical MV repair (65 versus 83%) and had worse outcomes.48 Patients with higher SEP were more likely to travel further for surgery and receive operations from higher volume surgeons.48
Data regarding transcatheter MV repair (MVR) compared to open MVR was queried from the National Inpatient Sample (NIS) in 2017.49 Patients undergoing TMVR were older, more affluent and 46% were women. There was no difference in adjusted mortality between surgical MVR and TMVR.50
Edge-to-edge Valve Repair
Access to TEER should be placed in a context that includes the burden of severe mitral regurgitation (MR), both primary and secondary. To date, only one single-centre study from England assessed racial differences in the prevalence of moderate or severe MR, with white patients having 6.7% and black patients 5.3%.51 In the US, black people have a disproportionate burden of hypertension, chronic kidney disease, adverse socioeconomic environment and structural racism that puts them at higher risk for incident heart failure and subsequent hospitalisations and death.52 It is therefore reasonable to extrapolate that the burden of severe secondary MR may be higher among black patients, but data supporting this is lacking.
Studies evaluating disparities in TEER access and outcomes have used the NIS from 2013 to 2018.53–56 The majority of TEER cases analysed in these studies were most likely to treat primary MR since the Centers for Medicare and Medicaid Services approved coverage for patients with secondary MR in January 2021. Rates of usage from 2013 to 2018 were significantly higher for white patients compared with black and hispanic patients (38 versus 29.7 versus 30.5 per 100,000 people over 65 years of age, respectively).55 Black patients were significantly younger, had a higher burden of comorbidities and up to 50% of them lived in the lowest MHI neighbourhood quartile.54,55 Even after adjustment of pre-procedural differences, black patients experienced a higher rate of death but a similar rate of overall in-hospital complications (composite of death, bleeding, cardiac and vascular complications).55 Similarly, those living in the lowest income quartile had worse in-hospital outcomes when compared with those in the highest income quartile.55 Additionally, urgent TEER was more frequently seen among younger patients, those with hispanic ethnicity, Medicaid insurance and receiving TEER at a small hospital or in the north-east region, and was associated with higher in-hospital mortality (4.5 versus 1.6%), prolonged length of stay (6 versus 2 days) and total costs (US$71,451.90 versus US$44,981.20).56
Socioeconomic Disparities in Usage and Resource Access in Left Atrial Appendage Occluder Device Placement
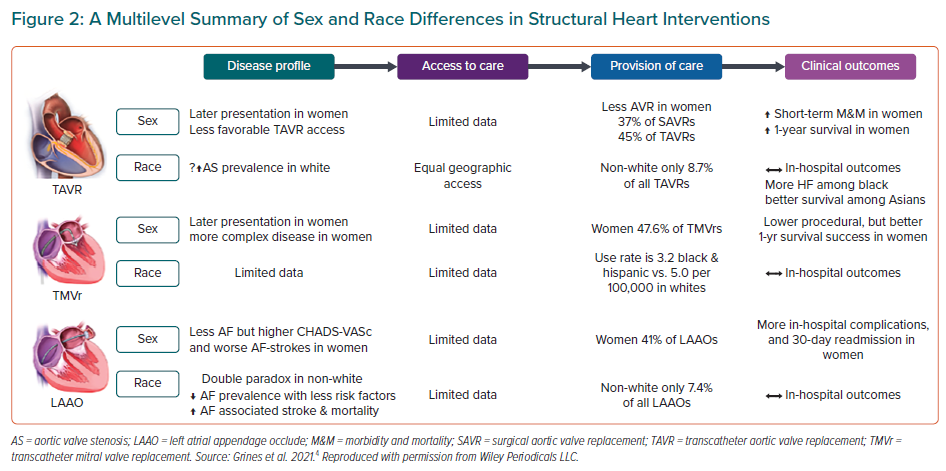
Using the NIS database, LAAO were placed in 6,458 patients between 2015–2018, of which 5% were in black patients and 40% were women.49 Based on census data, it was estimated that LAAO devices were used 2.3 times more frequently in white patients.49 MACE were more common in black patients (OR 1.6; CI [1.22–2.10]; p<0.001) compared to white patients, but no differences were found based on ZIP code income quartiles.49 Likewise, women were more likely to have complications despite adjustment for race and income.49 Figure 2 outlines a multilevel summary of sex and racial differences in structural heart interventions.53
Socioeconomic Disparities in Usage and Resource Access in Adult Congenital Heart Disease-related Percutaneous Repair Procedures
Despite the steady decrease in congenital heart disease (CHD)-attributed mortality in the US, black patients continue to experience higher age-adjusted mortality compared to their white counterparts.57 Similarly, a sex disparity disproportionately affecting men is also present.57 The reasons behind these differences are complex and may include gaps in care, travel distance, socioeconomic disadvantages, poor access to health insurance or low maternal education.58–62
To date, despite increasing use, disparities in access or outcomes of transcatheter therapies among adults with CHD have not been examined. Whereas large multicentre studies focused on this population failed to report on race or ethnicity.63–66 Data from the NIS 2011–2014 demonstrate that up to 40% of transcatheter pulmonary valve replacements were performed in patients who were not white.67 Additionally, there seems to be a similar sex distribution among patients receiving transcatheter pulmonary valve replacement as well as among patients with Ebstein’s anomaly who received valve-in-valve tricuspid valve replacement.68
Proposed Aetiology for Disparities in Access and Usage of Coronary and Structural Interventions
The aetiology for disparities in the access and usage of coronary and structural interventions are multifactorial. Limited access to quality healthcare is certainly a significant reason that is intrinsically linked to adverse SDOH in communities with low SEP.69 In the US, communities with a high proportion of uninsured or underinsured patients lack the presence of high quality well-funded hospitals or institutions that have expertise in performing coronary and structural interventions.69,70 Without a hub and spoke model that could reliably care for patients regardless of their insurance status, significant access barriers will remain in place. Government policies in the US have incentivised physicians to practice in rural and/or underserved communities through medical school loan repayment forgiveness and visa waiver opportunities.71 Despite this strategy, it is imperative to involve multiple stakeholders to secure the retention of these qualified physicians as opportunities for them and families may be limited in these areas. This limited access to high-level centres is more acutely felt in the field of adult CHD (ACHD) nearly half of the US population lives more than 1-hour drive from an ACHD centre and nationally there are fewer than 500 ACHD board certified physicians.59,72
SEP-related disparities in standards of care with patients not receiving guideline-directed structural and coronary interventions may also be the result of structural racism and sex biases. It may be perceived that patients of lower SEP may be more risk averse due to increased comorbidities, perceived increased risk of complications, such as bleeding, lack of adequate social and financial support after they have undergone coronary and structural interventions which often require close clinical follow-up, as well as adherence to prescribed antiplatelet and/or anticoagulation agents. It has been shown that physicians’ implicit biases on sex, race, ethnicity and other factors affect physician behaviour which results in differences in medical treatment and this perpetuates healthcare disparities.73 Black physicians did not show implicit preference for black or white patients. It has also been shown that women physicians are less likely to have implicit biases compared with male physicians.74 Race and sex concordance between physicians and patients has the potential to improve patients’ experiences and outcomes.75,76 Women, black and hispanic physicians are underrepresented in the cardiology workforce.77 This lack of sex, racial and ethnic diversity may also be another contributing factor in the disparities affecting patients with low SEP in coronary and structural interventions.
Other factors that may disproportionately affect patients in lower SEPs when deciding whether or not they will proceed with a coronary or structural intervention include the lack of support when having to take medical leave or time away from their work or home. This can range from the need to have a daily cash income in order to live day by day, lack of affordable childcare or safe, reliable public transportation.2
Lower health literacy in patients with lower SEPs may affect understanding and the way they cope with their underlying heart condition.2,22 This may be further worsened by lack of trust in the healthcare system and poor physician-patient communication.2,4 This is heightened among patients with lower SEP who do not speak the primary language of the community they reside in.2
Proposed Solutions to Socioeconomic Impact on Disparities in Access and Resource Usage
A multifaceted approach is necessary to address the socioeconomic impact on disparities in access and resource usage for coronary and structural cardiac interventions. The proposed solutions require interventions at multiple levels, such as at local and national government level, healthcare system level, community level and at the level of the individual and family.
Local and National Government
Interventions by the local and national government will require state and national legislation to institute policies that:
- Shift the focus from high-level advanced treatments to effective preventive strategies in communities with low SEP;
- Enable access to high-level treatments at high-volume high-quality centres regardless of insurance status;
- Improve funding, avoid penalties and improve quality at local hospitals that provide care for communities with low SEP.
Legislation at the state and national level is also required to implement sick leave and family leave policies for patients with SEP who have low-wage jobs that are paid by the hour. The ability of these patients to have sick leave and family leave would expand their ability to take the necessary time to address their or their family’s healthcare needs. The Affordable Care Act was effective in reducing the rate of uninsured non-elderly adult Americans since its implementation with the greatest reduction seen in patients with low SEP with annual household income of <US$36,000.78 However, there is still a need to elevate the quality of this health insurance since although coverage is provided, the amount of coverage offered may not allow access to quality expert care due to greater cost. Expansion of funding to these government-based insurance plans may increase access for communities with low SEP to these advanced coronary and structural interventions.
Healthcare systems
Increasing the presence of medical schools and large healthcare systems in communities with low SEP can help build bridges that reinforce primary and specialty care, allowing for timely referral to high-level centres. Increasing racial and ethnic diversity in the physician workforce within healthcare systems can also help decrease the SEP-related disparities in coronary and structural interventions. Additionally, interconnecting healthcare systems beyond insurance coverage is also a primordial step towards improving access to structural and ACHD interventions.
Community
Community-led initiatives may also assist in addressing the social factors limiting access to care for patients with low SEP. These interventions could focus on transportation solutions through car shares and more accessible public transportation with support from local governments or socially responsible companies. Subsidised programmes to assist in childcare and family care could allow patients who are primary caregivers to be able to have the time needed to address their healthcare needs including coronary or structural interventions.
Individuals/families
Implicit bias education for all members of the heart team can increase bias awareness and decrease SEP-related disparities.79 Use of standardised protocols have reduced bias in STEMI and/or cardiogenic shock populations.12 Physician training in effective physician-patient communication could improve interactions with patients and improve their trust and understanding of the physician’s recommendation with regards to structural and coronary interventions. This may also improve patients’ acceptance of these physician recommendations. Addressing the mistrust of healthcare providers and the healthcare system especially among black patients may improve some of the disparities seen in this patient population.80
Increased and improved patient education programmes through digital, print and audio-visual media could improve patient’s understanding of coronary and structural cardiac interventions and allow patients to make more informed decisions about their care.
Readily available access to language interpretation services may improve patient-physician communication for patients with low SEP who do not speak the primary language of the community in which they reside.
Conclusion
The burden of CV disease-related morbidity and mortality is felt greatest in patients with a low SEP. There are multiple reasons for these health disparities which include societal, healthcare system-related, community and individual reasons.2 Disparities in access and resource usage for coronary and structural interventions for patients with low SEP exist and the underlying reasons are multifactorial. These include structural racism leading to limited access to high-quality care and other adverse social determinants of health as well as implicit biases and ineffective communication. Addressing these disparities require a multi-layered approach at the level of national and local government, healthcare system level, community level and individual/family level.