Realtime 3D echocardiography (RT3DE) has been one of the most relevant advances in cardiovascular imaging in the last three decades. Since its initial description in the early 1970s, revolutionary progress in transducer technology and computer sciences have facilitated realistic 3D visualisation of the cardiac structures with high spatial resolution and satisfactory frame rates. RT3DE turned out to be crucial in providing new insights on the mitral valvular complex, enhancing operative and interventional approaches, and patient care. Mitral regurgitation (MR) is the most common valvular heart disease in the Western countries, affecting more than two million people in the US.1,2 The progression of regurgitation and the prognosis of the disease are largely determined by the aetiology and pathomechanism.3 The surgical trend toward personalised, tailored reconstructive operations and the emerging percutaneous techniques necessitate a deeper understanding of the pathophysiology and more accurate quantification of the severity of mitral insufficiency. The limitations of conventional 2D, cross-sectional echocardiography (2DE) in the accurate assessment of the localisation and extent of the anatomical and functional abnormalities of the mitral valve (MV) are partially overcome by volume-rendered RT3DE. In addition, 3DE has further advantages in grading the severity of regurgitation and in guiding percutaneous interventions. In this article, we aim to summarise the latest developments and current applicability of 3DE in the evaluation of mitral regurgitation.
Comprehensive Assessment of the Mitral Valve by 3D Echocardiography
3D imaging has undergone impressive improvements from the initial cumbersome reconstruction of 2D echocardiograms into 3D data sets, to the development of realtime 3D volume-rendered images. The currently used matrix-array transducers contain more than 3,000 piezoelectric crystals arranged in a grid configuration, allowing both live single-beat image acquisition, with or without colour Doppler, and electrocardiogram (ECG) triggered multiple-beat acquisition with higher spatial and temporal resolution. To take full advantage of 3D imaging, one needs to be familiar with the different methods of data acquisition, image optimisation and post-processing, the forms of post-acquisition display and the various cropping-slicing possibilities.4 After the basic 2D study, a systematic 3D evaluation of the entire MV complex is accomplished according to well defined protocols.5–7 The standard transthoracic and transoesophageal echo windows are used, but as a consequence of volumetric data acquisition considerably fewer probe manipulations and ultrasound rotational manoeuvres are required. Usually a biplane format is obtained first, which depicts the MV and the left ventricle (LV) in a reference plane and simultaneously in a second rotational plane with optional colour flow data. Realtime narrow sector display provides 30–60° pyramidal data sets with excellent spatial and temporal resolution for the initial assessment of the leaflets, commissures and chordae. By selecting an arbitrary region of interest, a truncated pyramidal data set is captured, called ‘wide sector focused’ or ‘zoom’ format. This is the most useful imaging mode for the prompt depiction of the main valvular pathology, since from the surgical en–face view it provides an exceptionally realistic portrayal of both leaflets. The full volume mode, which combines a series of narrow, wedge-shaped subvolumes acquired with ECG gating, is ideal for characterisation and offline analysis of the entire MV apparatus. The large acquisition sector and optimal spatial and temporal resolution (30–50 ms) of this mode enable thorough evaluation of complex lesions, but the emergence of embarrassing stitching artefacts renders it unsuitable in the case of cardiac arrhythmias. Full volume can be supplemented with colour Doppler, which fundamentally altered our conception on the spatial shape of regurgitant orifices and jets. The real beauty and challenge of 3DE comes after image acquisition. Image optimisation by thresholding and the removal of unnecessary parts by cropping, permits looking inside the volume data set focusing on the pathology of primary importance. Volume-rendered images of the valve obtained with this post-processing are ready for examination from unique perspectives previously unavailable with 2DE, and for further offline analysis with customised software.
New Insights into the Functional Anatomy of the Mitral Valvular Complex by 3D Echocardiography
The MV apparatus is composed of several structures; annulus, leaflets, chordae and papillary muscles.8 Optimal systolic closure of the valve and thus valve competence depends on the morphological integrity and ideal geometry of its components and the surrounding cardiac chambers. 3DE has contributed considerably to our understanding of the role of spatial interactions between the components promoting efficient valve closure. The mitral annulus (MA) has a non-planar, saddle-shaped, oval configuration,9 which can be characterised with several parameters by RT3DE (e.g. anteroposterior and intercommissural diameters, area, height and ellipticity).10 The two high points lie anteriorly and posteriorly on the short axis; the low points are located medially and laterally on the long axis. When seen from the left atrial perspective the anterior portion is a robust fibrous line of mitral-aortic continuity and the posterior portion is a predominantly muscular, C-shaped ring between the left atrium and ventricle. The complex saddle shape of the annulus confers advantages in minimising peak mitral leaflet stress.11 A normal annulus displays cyclic variation in size and shape during the cardiac cycle.12,13 The sphincteric action of the MA characterised by early systolic reduction in anteroposterior diameter and area, as well as deepening of the saddle shape, is conducive to optimal leaflet coaptation.14 RT3DE has been demonstrated to add relevant information about the differences in annular shape and function in various cardiac diseases.15 Functional and ischaemic MR is associated with annular dilation, flattening and reduced contractility.16,17 The asymmetrical shape distortion in ischaemic MR reflects the local remodelling of the LV due to the infarction. In myxomatous valve disease, the annulus is enlarged but remains dynamic, however, conformational changes do not optimally assist leaflet closure.18,19 In long-standing atrial fibrillation the loss of atrial contraction and remodelling result in blunting of phasic area change that might play a role in the development of MR.20
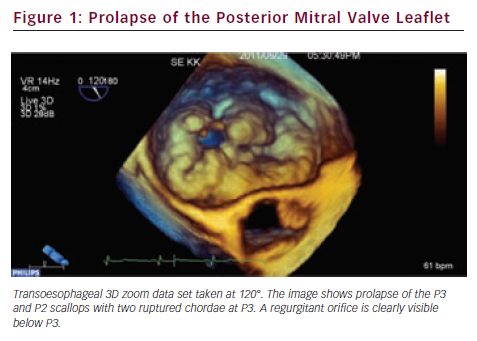
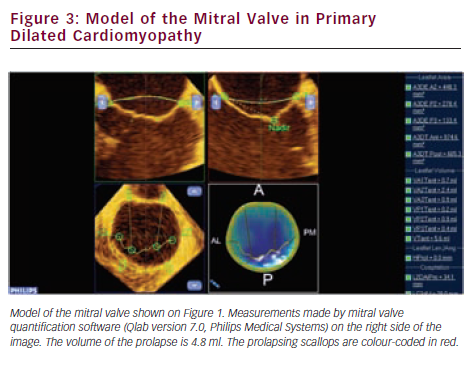
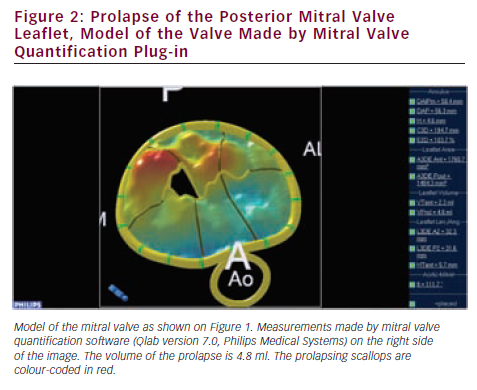
Since valve repair techniques have become preferred over valve replacement, information on the pathologic anatomy of the leaflets provided by echocardiography has become indispensable for patient care. The two leaflets of the MV have basal, clear and rough zones between the attachment point and free edges, and three scallops between the commissures.21 Each scallop may need to be managed separately by the surgeon contemplating valve repair. As a consequence of the variable appearance of both leaflets and the tomographic nature of traditional echocardiography, accurate segmental analysis of the leaflets by 2DE has major limitations and needs expertise in mental 3D reconstruction of the image. RT3DE is able to depict the valve from both the ventricular and atrial aspects, from where the pathology of any scallop is best appreciated. The left atrial perspective permits a rapid overview of the valve, because the entire surface of both leaflets is clearly visible from here. Turning the image upside down, the ventricular surface of the valve with the semilunar coaptation line and the insertions of chordae can be examined. With rotating and cropping manoeuvres, the valve pathology can be further interrogated from unique perspectives, which were physically unobtainable by conventional 2DE. For quantitative analysis, the 3D data set is cut in several simultaneous arbitrary cross-sectional planes using multiplanar reformatting (MPR) or slice mode and anatomically exact distance and area measurements are performed on these 2D-like slices. The possibility of free sliding and rotating of the cutting planes in the 3D volume may be particularly advantageous in determining the exact location of prolapse or flail, perforations or clefts in extensive and complex lesions. Besides, 3DE also provides colour Doppler flow data; hence the origin of the regurgitant jet and, therefore, the site of responsible valve abnormality can be easily determined. Although direct measurements in 3D images cannot be performed, vendors offer different 3D offline analysis software packages, which allow complex measurements such as 3D lengths, non-planar areas and volumes. MV quantification (MVQ) software creates static or dynamic models of the valve (see Figures 1 and 2). First the anatomical reference points are determined, then the annulus is outlined and the leaflet contour is traced manually or semi-automatically.22,23 These fascinating, colour-encoded, surface-rendered images serve as topographic maps of the leaflets and the valve apparatus and give valuable supplementary information to the surgeon before the inspection of the immobile valve in the bloodless heart during operation.24 MVQ calculates numerous standardised parameters including curvilinear length of the leaflets, 3D surface area of the individual scallops and the height and volume of a potential prolapse.22 With the combination of these geometric parameters, leaflet redundancy and displacement can also be characterised, which may improve planning complex, individual repair techniques.25,26
The diagnostic capabilities of 3DE in the assessment of the subvalvular apparatus are utilised more and more due to the increasing need to manipulate the chordae and the papillary muscles surgically. Optimal coaptation and apposition of the leaflets, necessary for valve competence, are provided by the support of primary tendinous chordae. Even minor variations in chordal branching and distribution or in the position of the papillary muscles may result in the reduction of the coaptation zone inducing regurgitation.27 Insufficient chordal support and undue stress on the leaflets may lead to degenerative, myxomatous transformation of the valve tissue enhancing prolapse.28 In functional mitral regurgitation changes in the spatial relationships between subvalvular structures cause malapposition of the free edges of the leaflets due to tethering.29 3DE is outstanding in the evaluation of such spatial relationships and it is suitable for detailed analysis of these pathogenetic mechanisms.
The Potential of 3D Echocardiography in Assessment of the Pathomechanism of Mitral Regurgitation
Continuous evolution of 3DE in the last five years has led to the recognition of this technique as the new clinical standard for the assessment of MV pathology and the modality of choice for surgical planning. There are several ways in which MR may develop, and the different mechanisms may also occur in combination, when more than one unrelated pathologies co-exist. The first, classic description of Carpentier divided valve dysfunction into three main categories regarding the opening and closing motion of the leaflets in relation to the annular plane.21 With the advent of transoesophageal echocardiography (TEE) in the early 1980s, functional classification of MR underwent further differentiation:
- type I, normal leaflet motion;
- type II, excessive motion;
- type III, restricted valve motion;
- type IV, systolic anterior motion (SAM); and
- type V, hybrid motions.30
Incremental diagnostic power of RT3DE became clearly visible in several specific areas including spatial analysis of prolapse and flail, identification of clefts and perforations, postsurgical assessment of residual regurgitation and paravalvular leaks.
Degenerative Mitral Valve Disease
Precise identification of the anatomic lesions, which lead to valve dysfunction in degenerative mitral valve disease, is essential in planning surgical repair and profoundly affects success rate. The significant improvements in image quality with matrix transducers and the availability of volumetric quantification software have rendered 3DE the standard of clinical care from disease evaluation, through surgical planning to post-repair follow-up. Degenerative valve disease comprises different forms and aetiologies of prolapse, such as Barlow disease (BD), fibroelastic deficiency (FED), forme fruste and Marfan syndrome, and specification of the underlying disease process have implications in terms of complexity of successful valve repair.31 Although differentiation between the two main subgroups of BD and FED usually can easily be performed based on 2D morphologic features, such as annular size, shape and calcification, leaflet thickening and extension of prolapse, in 20 % of the cases the correct diagnosis remains a challenge. In this subset of patients, several 3D parameters including volume and height of the prolapse help define the aetiology.32,33
Accurate delineation of the pathoanatomy of leaflets, localisation of the prolapse and identification of flail segments are the main tasks of the pre-operative study. There is a substantial body of literature confirming that 3DE has an unquestionable superiority over 2DE in this regard, having more than 95 % accuracy with RT3D transoesophageal echocardiography.34,35 The accuracy of RT3D transthoracic echocardiography (TTE) is similar to 2DTEE; hence it represents a reasonable alternative to the semi-invasive procedure.36 The added value of 3DE is specifically significant in complex lesions with extensive multisegmental or commissural prolapse.37–40 3DE may identify small areas of prolapse that are not readily apparent to surgeons inspecting the valve in an immobile, non-physiologic state.41 With MPR dominant prolapsed segments can be accurately differentiated from secondary prolapsed segments, providing valuable, subtle details on morphology to the surgeon.42 Complex lesions involving the entire posterior leaflet or the commissures, lesions of the anterior leaflet and bileaflet prolapses are more precisely delineated by 3DE. This can help to match the skills of the surgeon to the adequate repair technique. Intraleaflet asymmetry necessitating surgical correction is almost exclusively detected by 3DE. Whenever valve dysfunction is complex and MR has more than one substrate (e.g. cleft or perforation combined with prolapse), realtime 3D anatomy with multiplane reconstruction of the jet can help to identify the multiple origins and to discern their relative contribution to the severity of the lesion.43,44
Use of 3D Echocardiography in Surgery
Beyond the initial assessment of the basic pathophysiological triad of aetiology lesion dysfunction in degenerative MV disease, 3DE has a pivotal role in choosing the optimal surgical treatment. The global characterisation of the valve morphology in terms of annular enlargement, planarity, dynamicity, coaptation geometry and leaflet redundancy defines useful parameters for guiding surgery. The most useful parameters offered by 3D quantification software are the 3D area and axes of the annulus, the curvilinear length of A2 and P2 scallops, the 3D leaflet surface areas and the distances between the commissures and papillary muscles.45 The different patterns of annular distortion revealed by 3DE have provided inspiration for annuloplasty ring design. The most recent annuloplasty rings are disease-specific and geometrically shaped symmetrical or asymmetrical to accommodate the underlying pathology.46 The selection of the proper ring size and model is facilitated by 3D measurements. Information on the tissue redundancy of the prolapsing leaflet can guide the surgeon in deciding the extent of MV resection. In this regard, curvilinear leaflet length, exposed leaflet surface area and volume of the prolapse measured scallop-by-scallop are key parameters confirming the clinical potential of the 3D technique.25,47,48 Post-repair systolic anterior motion (SAM) of mitral leaflets and subsequent dynamic outflow tract obstruction may occur in 10 % of patients, due to anterior malposition of the coaptation line. Echo predictors of increased risk for SAM, such as the distance between the septum and the coaptation line and the length ratio of the mitral leaflets, are well defined by 2DE.49,50 Since the phenomenon depends on spatial relationships, 3DE may have incremental value in its recognition. Valve repair is the treatment of choice in degenerative MV disease and it is recommended even in a subset of asymptomatic patients with severe regurgitation, where the probability of a successful repair is high.51,52 3D echocardiographic parameters of prolapsing height, anterior leaflet surface area and multi-segment or bileaflet prolapse have proven to be strong predictors of repair complexity.53 A mismatch between the surgeon’s expertise and the complexity of repair may lead to residual or recurrent MR or a need for valve replacement. By targeted referral practice, based on objective appraisal of repair complexity, the general repair rates of 60–70 % could be increased.
Functional Mitral Regurgitation
In functional MR, the structural integrity of the chords and leaflets remains intact, but in consequence of the local or global remodelling of the LV, the dyssynchronous myocardial contraction and the distortion of the hypodynamic annulus, leaflet tethering develops and the reduced coaptation induces MR. The restoration of the coaptational surface is the ultimate, common goal of all repair techniques and RT3DE is the first imaging modality to measure coaptational length and surface.54 Numerous 2D parameters have been described to measure the extent of leaflet tethering (tenting height, tenting area) and the symmetric or asymmetric pattern of traction exerted on the leaflets by second order chordae,55 but all of them are dependent on the ultrasound interrogation plane. The parameters measured by RT3DE are much more accurate and tenting volume has proven to be the single major determinant of the regurgitant orifice area (see Figure 3).56 The astonishing ability of the leaflets to increase their surface area in response to chronic tethering was revealed by 3DE. This form of active adaptation can potentially provide therapeutic opportunities in the future.57 Annular and subvalvular remodelling that is manifested by dilation and shape distortion of the annulus, and displacement of the papillary muscles altering axial relationship of the chordae and the leaflets varies extensively between patients.58 New, innovative subannular surgical therapies, such as strut chord transection, papillary muscle repositioning or infarct plication, should be increasingly based on the 3D quantitative evaluation of the left ventricular geometry in the individual patient. Changes of the LV shape and dyssynchrony during exercise characterised with 3D sphericity and dyssynchrony indices are predictors of the dynamic worsening of MR in heart failure patients.59
Rare Aetiologies of Mitral Regurgitation
A detailed discussion of the whole spectrum of congenital MV disorders is far beyond the scope of this article. Nevertheless, the use of 3DE60 can be abundantly demonstrated through the evaluation of mitral clefts. A cleft mitral valve is usually associated with other cardiac malformations, but may also be present in isolation. Isolated clefts are easily missed by 2DE,61 and should be suspected in cases of unexplained MR or discrepancy between morphological and colour Doppler findings.43 3DE is superior in the detection and localisation of clefts and provides additional information on the depth and width of the lesion and the presence of fibrosis or edge retraction.62 The cleft usually involves the anterior leaflet, and may be associated with abnormal chordal attachment to the interventricular septum leading to outflow tract obstruction. The presence of accessory papillary muscles and malposition of the papillary muscles have been described with posterior leaflet clefts. The co-existent anomalies of the subvalvular apparatus may easily be depicted and analysed with full volume 3D data acquisition. Through the 3DE, it is becoming increasingly apparent that prolapse and myxomatous changes of the leaflets may be combined with clefts, which may request complex repair techniques. A caveat of the improved visualisation of the valve by 3DE is the misinterpretation of normal splits and indentations as clefts.
New Perspectives in the Quantification of Mitral Regurgitation with 3D Echocardiography
Irrespective of aetiology and symptoms, severe MR seriously impacts on the long-term outcome of patients in terms of atrial fibrillation, heart failure, sudden cardiac death and overall morbidity and mortality.63 In the era of rapidly developing surgical and interventional repair techniques, the accurate and quantitative grading of MR has become the paramount goal of cardiologists seeking proactive patient management. 2D echocardiography holds inherent inaccuracies due to several false geometric and haemodynamic assumptions in flow convergence methods and the large number of measurements needed in quantitative Doppler. Accordingly, even with the integrated approach recommended by the current guideline, grading MR remains a challenge.64 At present, emerging methods of MR quantitation by 3DE involve the assessment of the cross-sectional area of the vena contracta (VCA), delineation of the real anatomic regurgitant orifice area (AROA) and the measurement of the factual proximal isovelocity surface area (PISA) for the calculation of the effective regurgitant orifice area (EROA).
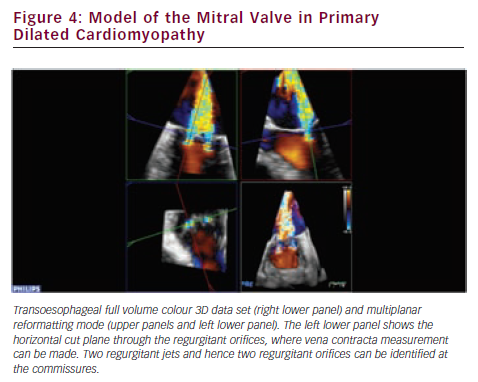
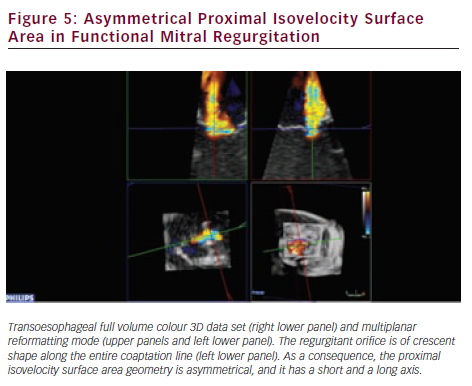
RT3DE provides unlimited imaging planes in the volume data set. Therefore, 3D-guided planimetry of the narrowest portion of the regurgitant colour flow jet can easily be performed. VCA is a highly feasible65–67 validated indicator of MR severity.68,69 In functional MR VCA shows typical elongation along the coaptation line, caused by leaflet angulation due to chordal tethering. A broad range of irregular asymmetries characterises the VCA in myxomatous valve disease, which also highlights the inadequacy of vena contracta width measurements by 2DE. Direct visualisation of VCA is based on conventional 3D colour Doppler imaging (see Figure 4). However, other novel approaches established on laminar flow characteristics are also available to assess VCA and the flow volume inside it. Using a multibeam high-pulse repetition frequency 3D colour Doppler, semi-automated VCA measurement from the back scattered Doppler power is possible.70,71 Dealiasing of colour Doppler flow at the vena contracta allows for direct quantification of MR flow volume in the region.72 VCA is smaller than the true anatomic regurgitant orifice in proportion to the flow contraction, which is determined by the unique geometry of the orifice. RT3DE derived AROA provides an adjunctive useful method to quantify MR severity.32 Manual tracing of the leaflet edges makes the computation of the 3D minimal surface area of non-planar orifices possible, which is particularly advantageous in complex MV pathology (e.g. in case of flail or overlapping leaflets). Although traditional 2D flow convergence, the so called PISA method, is now by far the most recommended quantitative approach for MR grading; it suffers from numerous erroneous geometric and haemodynamic assumptions leading to substantial under- or overestimation of EROA.73 The evolution of 3DE has brought significant refinements to this field. Firstly, Yosefy and colleagues used a hemielliptic surface area formula for EROA calculation requiring three axial measurements, which showed much better agreement with the quantitative Doppler reference method.74 Recognising that even in functional MR, diverse PISA shapes (e.g. ‘mountain’ or ‘valley’ type) can be differentiated; Matsumura and colleagues have developed a more sophisticated hemiellipsoidal programme that can be used for all types PISA geometry.75 In fact, the real PISA surface is neither hemispheric nor hemiellipsoid, but always displays a unique shape according to the individual anatomic orifice that creates it. An ideal echocardiographic method would embrace the PISA’s true 3D complexity (see Figure 5), which continuously changes during systole, adding the crucial fourth dimension to EROA analysis. In the last few years, cutting edge ultrasound technologies took significant steps towards this. First 3D PISA images were sliced sequentially and the surface contour traced manually to eliminate all geometric approximation. The method was later automated using the Halcon software.76 Other systems demand only two reference points in the 3D colour Doppler data set, then the fully automatic segmentation and computation of the isovelocity surface area is performed.77 These methods need instantaneous, ‘single-beat’, full volume colour Doppler image acquisition at high frame rates, which is the latest brilliant achievement of the 3D technology.78
Limitations, Future Directions of 3D Echocardiography
3DE shares the common limitations of the 2D technique originating from the physical properties of ultrasound technology, including side lobe, drop out and attenuation artefacts. Temporal and spatial resolution is still an issue. As a consequence, visualisation of thin, rapidly moving objects in the far field of the ultrasound beam, such as the leaflets of the MV or vegetations may be compromised, especially in obese patients with a poor echo window. ECG-gated, reconstructed images contain stitching artefacts, which disturb the distance and area measurements. The intra- and inter-observer variability of the measurements will improve with continuously evolving software offering more and more automated computations. 3D display of the volumetric data by holographic or stereoscopic visualisation promises to be possible in the near future.
Conclusion
3DE represents a breakthrough new technology in the assessment of the mitral valvular complex and the exploration of the aetiology and pathomechanism of MR, and it will likely improve the quantification of the regurgitation. The imminent incorporation of 3DE into society guidelines will further promote its widespread use in the clinical routine.