Cardiovascular diseases are the leading cause of mortality throughout the world including Spain, mainly in the form of ischaemic heart disease.1–3 Acute coronary syndrome (ACS) is one of the most severe forms of presentation of ischaemic heart disease. Coronary atherosclerosis is the most frequent pathophysiological process underlying ACS, which is complicated by atherosclerotic plaque rupture or erosion.4
A range of factors have been reported to contribute to the development of ACS.5 The classic risk factors related to the development of cardiovascular diseases are smoking, arterial hypertension, dyslipidaemia and diabetes. In addition, many epidemiological studies have found an association between environmental pollution exposure and harmful health effects, with an important increase in morbidity and mortality.6 The existing evidence indicates that most deaths caused by environmental pollution are attributable to cardiovascular diseases.7 This has led to the suggestion that environmental pollution constitutes a new modifiable cardiovascular risk factor.8
Air pollution consists of a complex mixture of compounds in the form of gases and particulate matter (PM), with evidence supporting PM as the main cause of cardiovascular morbidity and mortality.6 The precise mechanisms whereby the inhaled particles produce their adverse effects upon the cardiovascular system are not fully clear.9 After entering the body through the respiratory system, inhaled PM may trigger an inflammatory response at pulmonary level, with a secondary systemic inflammatory response that in turn exerts its cardiovascular effects.10
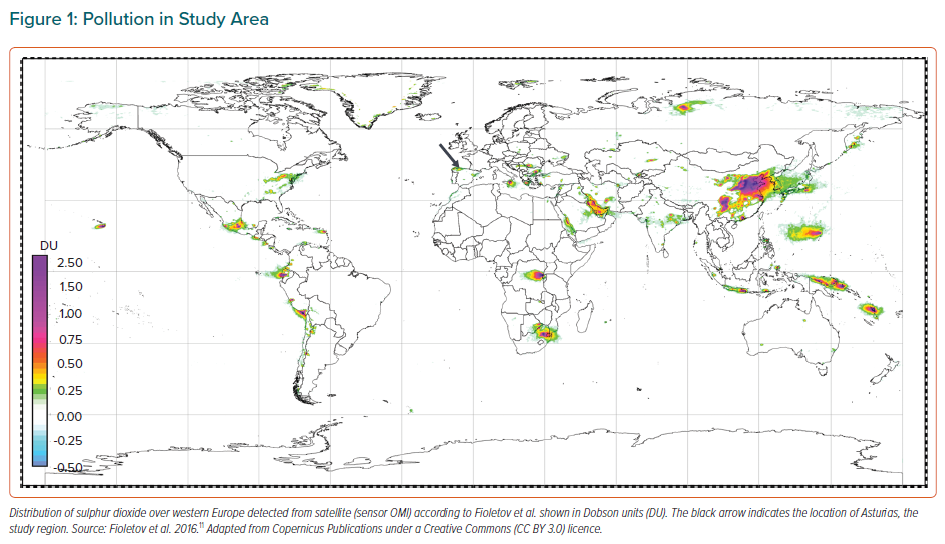
This study was carried out in Asturias in northern Spain, a region where coal burning and heavy industry have regularly been sources of sulphur dioxide and other pollutants. Satellites often detect high loads of sulphur dioxide over this region (Figure 1).11
Considering the pollution levels in the Asturias region, we hypothesise that exposure to ambient air pollution days before an acute MI could influence the observed white cell inflammatory response and the infarct size.12
Therefore, this study analyses the influence of exposure to environmental pollutants on the white cell inflammatory markers in patients with acute MI, evaluates whether such exposure influences infarct size and reviews current data in the literature.
Methods
Study Population
A prospective study was carried out involving 236 patients consecutively admitted to Hospital Universitario Central de Asturias (HUCA) from 1 January 2014 to 31 December 2015 with a diagnosis of ST-segment elevation acute coronary syndrome (STEACS), with successful (final TIMI flow 3) primary angioplasty/percutaneous coronary intervention (PCI). STEACS was defined according to the European Society of Cardiology clinical practice guidelines as persistent chest pain or other symptoms indicating ischaemia and ST-segment elevation on at least two contiguous electrocardiographic leads.13
Twenty patients were excluded due to sepsis, surgery or trauma in the previous 3 months, neoplasms, cirrhosis, chronic inflammatory or autoimmune diseases, active corticosteroid treatment, a life expectancy of less than one year or unsuccessful angioplasty (final TIMI flow 0, 1 or 2). The final cohort consisted of 216 patients.
Primary Angioplasty
Conventional coronary angiography was performed using radial or femoral artery access. The angiograms were evaluated based on the modified 17-segment model proposed by the American Heart Association, which includes the major coronary artery trunks and their main branches.14 An obstructive lesion was defined as a reduction of >70% in vessel diameter, except in the case of the left common trunk, where the percentage was defined as >50%. PCI was performed as established in the clinical practice guidelines.13
Study Variables
With regard to air pollutants and meteorological variables, we analysed daily mean values (24 hours) from the day before (lag 1) until 7 days before admission (lag 7) of each of the 216 patients admitted with ACS in the period 2014–2015. These mean values were calculated based on the data recorded in one of the four air quality monitoring stations in the municipality of Oviedo. Data were supplied by the local authorities (Consejería de Fomento, Ordenación del Territorio y Medio Ambiente). The selected station was Palacio de Deportes (43° 22' 03.1" N; 5° 49' 54.4" W), which is in an urban area. A nearby highway with heavy traffic clearly influenced the recordings. The access to the city through this highway was shut down for several days in December 2015 because of high concentrations of PM10 (particles ≤10 μm diameter that can be inhaled).
For the air pollutants, we analysed the data for sulphur dioxide (SO2), nitric oxide (NO), nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), benzene (C6H6), toluene (C7H8), xylene (C8H10) and PM. For meteorological variables, we recorded precipitation (in mm), temperature, pressure, relative humidity and wind speed.
A number of clinical variables were compiled for the patients, including cardiovascular risk factors (age, smoking, arterial hypertension, hypercholesterolaemia and diabetes), sex, kidney disease and previous MI. For the laboratory test parameters, we recorded haemoglobin, leukocytes, neutrophils, lymphocytes and platelet counts from the first blood count made after symptom onset (generally upon patient arrival in hospital), as well as ultra-sensitive troponin T peak concentration.
The following coronary angiographic data were recorded: culprit artery; number of diseased vessels; treatment of some vessel apart from the culprit artery; stent placement (or not); the number of stents placed; and final TIMI flow.
Primary Endpoint
The primary endpoint of this study was to investigate the impact of the exposure to ambient air pollutants on white cell inflammatory markers in patients with acute MI, and evaluate whether such exposure influences infarct size.
White cell inflammatory activity was assessed based on the neutrophil:lymphocyte ratio (NLR; absolute counts). Infarct size was analysed based on the median peak troponin value. For the purpose of the analysis, a large enzymatic infarct size was defined as infarction with peak troponin values above the median, while a small enzymatic infarct size was defined as infarction with peak troponin values below the median.
Statistical Analysis
The relationship between air pollutants an myocardial infarct size was studied by an analysis of time series. Continuous variables were reported as the mean ± SD or as the median and interquartile range (IQR) in the absence of normal data distribution. Qualitative variables were reported as absolute values and percentages. Ambient air pollution values for each patient were calculated as 24-hour averages from the previous day up to 7 days before admission.
For the purpose of the analysis, patients were divided into two groups of 108 according to infarct size, based on the median peak troponin value (2,991.5 ng/ml). The baseline characteristics of the two groups of patients were compared using the χ-squared test for categorical variables. Continuous quantitative variables with a normal distribution were compared using the Student’s t-test, while the nonparametric Mann–Whitney U-test or Kruskal–Wallis test was used in the absence of a normal distribution.
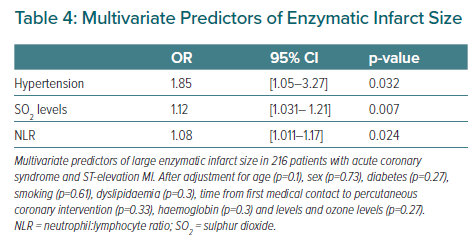
A multivariate analysis was performed based on a regression model in which the dependent variable was infarct size and the independent variables were the parameters found to be significant (p<0.05) in the univariate analysis. The results were expressed using the OR and 95% CI. Statistical significance was considered for p<0.05 in all cases. The SPSS statistical package was used throughout.
Results
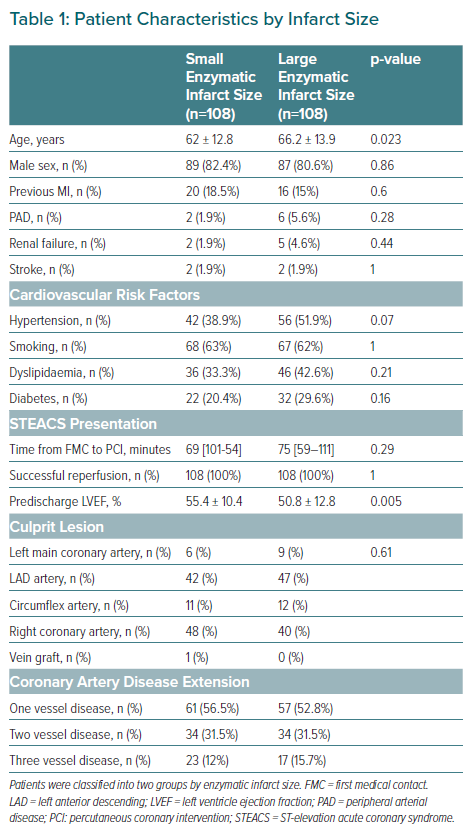
A total of 236 patients were recruited, of whom 216 had data finally analysed after applying the exclusion criteria. The baseline characteristics of the two groups are shown in Table 1.
There were significant differences between the two groups in terms of age and ventricular function at discharge. The patients with extensive infarcts were comparatively older and exhibited poorer ventricular function. There were no statistically significant differences between the two groups in terms of the prevalence of cardiovascular risk factors, culprit lesion or the extent of coronary disease.
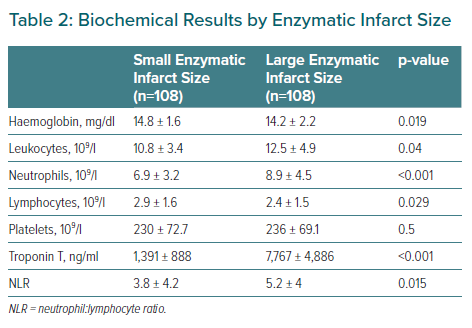
The laboratory test parameters are reported in Table 2. Patients with larger MIs presented higher neutrophil:lymphocyte ratios, leukocyte and neutrophil counts but lower lymphocyte counts than patients with smaller MIs.
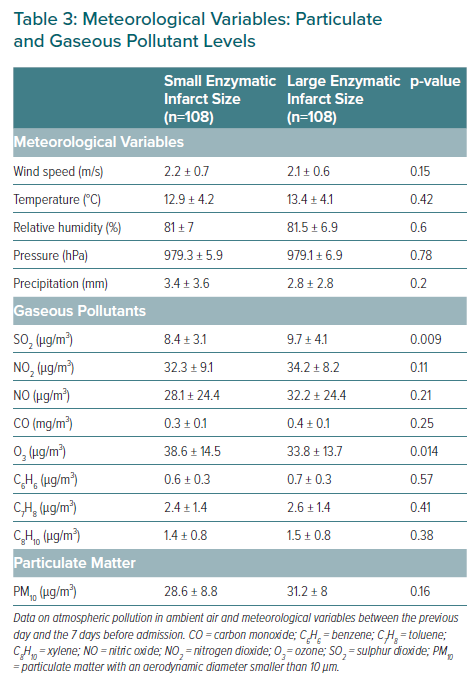
The meteorological variables and the levels of particulate and gaseous pollutants are shown in Table 3. No statistically significant differences were observed with regard to the meteorological variables or airborne particles. However, patients with larger MIs had been exposed to higher levels of sulphur dioxide and to lower levels of ozone in the 7 days before to admission. In the multivariate model, arterial hypertension, the sulphur dioxide levels, and the neutrophil:lymphocyte ratio were identified as independent predictors of infarct size in patients with STEACS (Table 4).
Discussion
The main finding of our study is the association found between sulphur dioxide, white cell inflammatory markers and infarct size in patients admitted due to STEACS treated with successful PCI. Patients with large enzymatic MIs presented with higher neutrophil:lymphocyte ratios.
Epidemiological Data
Air pollution is the single largest environmental health risk worldwide, with air pollution alone causing a significant number of deaths per year (one in eight of all deaths). In 2016, air pollution was the second most important risk factor for non-communicable diseases worldwide and 91% of the world’s population was exposed to harmful levels of air pollution. This mortality effect of air pollution is mainly due to heart disease and stroke, which are also the leading causes of mortality and morbidity worldwide. Therefore, by reducing air pollution, we could reduce morbidity and mortality from cardiovascular diseases.15
Air Pollution Particles
Air pollutants include gaseous pollutants (such as carbon monoxide, nitrogen oxides, ozone and sulphur dioxide) and PM. PM has been linked to a number of cardiovascular diseases; in particular, it seems that PM2.5 (particles ≤2.5 μm diameter), which comes from traffic and industry, and ultrafine particles (UFPs) are the most closely linked, as both can more easily reach the smaller airways and alveoli, and UFPs can even cross the alveolar-capillary membrane and spread through the systemic circulation to distant organs.
Air Pollution and Cardiovascular Disease
Several pathways have been proposed by which PM cause cardiovascular disease. One of them is the direct pathway, i.e. when PM2.5 and especially UFP move into the bloodstream and reach remote organs.
Due to the size, charge and chemical composition of UFP, it is much easier for them to pass through the lung epithelium and the alveolar-capillary barrier than larger particles. This exposure, even at low concentrations, can lead to particulates entering the bloodstream to reach a remote organ and potentially cause cumulative toxicity.
This translocation of UFPs to the bloodstream has detrimental effects on the cardiovascular system. After deposition on the vascular endothelium, UFPs can aggravate local oxidative stress and inflammation, resulting in atherosclerotic plaque instability, and may eventually lead to thrombus formation, leading to ACS.
Indirect pathways include oxidative stress at the pulmonary level and the inflammatory response, as well as specific pulmonary receptors that would activate the autonomic nervous system.16 Systemic inflammation is a risk factor for the progression of atherosclerosis, and pro-inflammatory mediators are strongly associated with increased blood coagulability and endothelial dysfunction, which may exacerbate myocardial ischaemia.
Several studies in the US and Europe have indicated high numbers of hospital admissions due to ischaemic heart disease related to higher PM levels, and a meta-analysis in 11 European cohorts of the ESCAPE project confirmed that long-term exposure to PM is associated with the incidence of coronary events, and this association persists at exposure levels even below the current European limit.17–19
When ambient particles interact with atmospheric gases (ozone, sulphur, nitric oxide and carbon monoxide), they generate secondary particles that are also associated with cardiovascular disease. For example, in a study by Li et al., a 10 μg/m3 increase in 2-day mean concentrations of PM10, SO2 and NO2 was significantly associated with increases in daily coronary heart disease mortality.20
Relation of Air Pollution and MI
In the present study, patients with larger MIs had been exposed to higher sulphur dioxide levels and to lower ozone concentrations. Sulphur dioxide emissions are linked to the combustion of coal and other heavy fuels for power generation and in industrial activities.
Domínguez-Rodriguez et al. found that patients admitted because of ACS and obstructive lesions had higher sulphur dioxide levels.21 Sulphur dioxide is a gas that at high concentrations can give rise to an inflammatory response at lung level, followed by proinflammatory cytokine release into the systemic circulation, causing endothelial damage and increasing the risk of atherosclerotic plaque rupture and consequent thrombosis.22 Likewise, other studies have reported a direct relationship between environmental sulphur dioxide levels and blood concentrations of proinflammatory and proatherogenic parameters.23
Infarct size is of great prognostic relevance in patients with STEACS. High-sensitivity cardiac troponin T (hs-cTnT) has become the biomarker of choice in assessing myocardial damage in the context of ACS. Its peak concentration has been shown to be a robust indicator of infarct size; as a result, it is widely used in clinical practice. Other biomarkers, such as N-terminal pro-brain natriuretic peptide (NT-pro-BNP) and the NTR (used in our study) are also independent predictors of infarct size in the same way as troponin.24 The NLR relates neutrophils (which reflect the immune response) and lymphocytes (which reflect the adaptive immune response). This parameter has been shown to have greater prognostic relevance than absolute leukocyte count in terms of patient mortality following STEACS.25,26 In our study, the patients with more extensive infarcts had higher NLRs, in concordance with the aforementioned studies.
Limitations
Our study has several limitations that must be considered. This is a non-randomised, observational study and our results must be considered hypothesis generating, which can potentially constitute the basis of broader investigations.
An inherent problem common in studies that analyse the effects of air pollution is that we cannot exclude the existence of errors in the measurement of exposure due to differences between what is measured by the sampling stations and the actual exposure of each person in a population (interindividual variability).
The climate of a region depends on various geographical factors such as latitude, relief and environment; therefore, the results of our study should be verified with those obtained in other geographical areas with different climates from those analysed in this study.
Finally, infarct size was not measured by cardiac MRI, which is considered the gold standard for the assessment of myocardial damage and ventricular volumes.
Conclusion
Patients with extensive MIs presented higher levels of white cell inflammatory biomarkers and had previously been exposed to higher sulphur dioxide concentrations than patients with smaller MIs.