Cancer and cardiovascular disease (CVD) are among the leading causes of death worldwide. In recent decades, there has been remarkable progress in the detection and treatment of cancer and CVD that has translated to a significantly improved prognosis for both conditions.1,2 Unsurprisingly, these trends have resulted in a growing population with both CVD and cancer. The conditions frequently coexist and share multiple mutual risk factors. Furthermore, cancer treatments share various detrimental effects in common, especially upregulation of cardiovascular risk factors.3 In the cancer population, CVD is the second most common cause of morbidity and mortality, second only to recurrent malignancy.4 In fact, the risk of CVD in the cancer population is 800% higher than that of the general population and the relative risk of coronary artery disease is over 1,000% more in cancer survivors compared to their cancer-free siblings.4 The overall burden of CVD in the cancer population is likely to increase with an increasingly ageing population and a high lifetime risk of both conditions in the developed world.
In response to the growing population of cancer patients and survivors with CVD the sub-speciality of cardio-oncology was developed to optimise care. Cardio-oncology is a relatively new sub-specialty within cardiology focusing on the optimal diagnosis, prevention and treatment of the cardiovascular consequences of cancer and its treatment. The world’s first cardio-oncology unit was built in the US in the MD Anderson Center in 2000. The international cardio-oncology society was created in 2009 and the first dedicated cardio-oncology service was started in the UK at the Royal Brompton Hospital in 2011.5,6 In a 2016 position document, the European Society of Cardiology provided a breakdown of cardiovascular complications of cancer therapy into nine main categories:
- left ventricular systolic dysfunction;
- arterial hypertension;
- pulmonary hypertension;
- valvular disease;
- cardiac arrhythmias;
- thromboembolic disease;
- peripheral vascular disease and stroke;
- coronary artery disease; and
- pericardial disease.
Palpitations are defined as a rapid pulsation or abnormally rapid or irregular beating of the heart. Patients often describe palpitations as a rapid fluttering in the chest, a skipped beat, or a pounding sensation in the chest.7 Palpitations are one of the most common presenting complaints when patients attend the emergency department or see their primary care provider or cardiologist.8 Palpitations are commonly benign and represent an abnormal awareness of one’s heartbeat. However, less commonly they represent abnormal heart rhythms. In the cancer population, palpitations are a frequent occurrence and investigations and treatment are comparable to that in the general population although with some nuances.
The sensation of palpitation can arise from extrasystoles or tachyarrhythmias. Less commonly, bradycardias can also be implicated. In patients who describe palpitations as a brief extra strong beat in the chest, it is thought that this is induced ventricular/atrial extrasystoles. In rapid persistent palpitations, it is thought that supraventricular tachycardias are implicated. This review will appraise the literature with regards to the development and management of palpitations in people with cancer. Specifically, this article will focus on the differential diagnosis, clinical approach, investigations and management of palpitations.
Diagnosis and Investigations
Aetiology/Differential Diagnosis of Palpitations
Palpitations can be divided into four categories for the purpose of this discussion:
- Cardiac causes.
- Anxiety disorders.
- Miscellaneous causes, such as medications/thyrotoxicosis, caffeine and anaemia.
- Unknown causes (16% of scenarios).9
Cardiac arrhythmic causes of palpitations are the most worrying for patients and they can be life-threatening. Table 1 demonstrates the breakdown and subcategories of palpitations. Nuances do exist in the cancer population, where patients are more likely to suffer cardiac palpitations due to cancer or complications of its treatment, as well as being more likely to suffer palpitations due to anxiety and stress from dealing with cancer and its treatment.
Clinical Work-up
The diagnosis of palpitations can be challenging for a variety of reasons, and their transient nature is a large contributing factor. Work-up can often be fruitless and patients are frequently left without a probable diagnosis and are not given any treatment.10 This means a large proportion of patients continue to suffer recurrences of palpitations, which can be a cause of morbidity and reduced quality of life. The current investigation process for palpitations is often guided by the treating clinician’s practice. There is little in the literature that could be used as a specific guidelines/policy document to guide practice.11 Clinical work-up will usually incorporate a combination of history, examination and subsequent investigation as required.
History
A good clinical history remains at the core of assessment and can help delineate high-risk from low-risk patients and it can guide investigations depending on the risk profile. A good history can provide more clues towards the diagnosis than the examination because palpitations have often spontaneously ceased by the time of the assessment. The initial clinical assessment is often used to paint a clinical risk profile of a patient and estimate the underlying likelihood of serious causes of arrhythmias. The pre-test probability is the single most important tool in building a risk profile for patients. In one study among patients with cardiac disease, palpitations were attributed to an arrhythmogenic focus 91% of the time.12 Therefore, the complaint of palpitations should be taken more seriously in patients with pre-existing cardiac disease. In a systematic review, the strongest predictive factors for significant cardiac arrhythmia were a known history of cardiac disease (likelihood ratio [LR] 2.03; 95% CI [1.33–3.11]), and palpitations affected by sleeping (LR 2.29; 95% CI [1.33–3.94]) or while at work (LR 2.17; 95% CI [1.19–3.96]). Strongly negative predictive factors included palpitations lasting less than 5 minutes (LR 0.38; 95% CI [0.22–0.63]) and a known history of panic disorder (LR 0.26; 95% CI [0.07–1.01]).13
It is vital for the clinician to establish duration and frequency of provoking/relieving factors and it may be useful to allow the patient to tap or clap out their perceived palpitation. Table 2 demonstrates the salient points in a palpitation’s history. It is important to search for red flags, such as syncope, exercise-induced palpitations or a family history of sudden death. It is important to elucidate what medication the patient is on as some chemotherapeutic agents are heavily implicated as arrhythmogenic substrates.
Examination
The role of the examination is limited as frequently it is performed when the patient is asymptomatic and there are no rhythm abnormalities. The examination should focus on any possible cardiac or systemic illness that may be implicated in palpitations. A good examination should look for evidence of cardiac disease in the form of signs of heart failure, an irregular pulse or valvular disease. Signs of non-cardiac causes of palpitations can also be elicited in the form of tremors/goitre (thyrotoxicosis) or pallor (anaemia). The usefulness of these clinical signs is unproven; however, as the data are largely limited to anecdotal evidence and there are currently no data evaluating the presence of these signs with arrhythmia. The only sign with good evidence is the presence of resting bradycardia (<60 BPM; LR 3.00; 95% CI [1.27–7.08]).14 While necessary during work-up, it is well recognised that clinical examination alone is not sufficient to accurately exclude clinically significant arrhythmias in most patients.
Clinical Investigations
There is a similar set of investigations that all oncology patients presenting with palpitations should undergo, which includes:
- Full blood count, urea and electrolytes (including magnesium and calcium) and thyroid function tests.
- Echocardiogram to identify structural heart disease that increases the index of suspicion for cardiac arrhythmia.
- 12-lead ECG with subsequent ambulatory monitoring dependent on the frequency of symptoms.
In the general population the above may be more than sufficient to reassure both the patient and the clinician regarding the benign nature of palpitations. However, the cancer population is deemed to be at high risk of CVD and therefore aggressive investigation is advised. It is advisable to undertake ambulatory monitoring tailored to the patient’s symptom burden and preferences. Ambulatory monitoring can be diagnostic when it establishes a correlation between palpitations and an ECG recording.15 In patients who experience asymptomatic arrhythmias the role of ambulatory monitoring is more tenuous in diagnosing palpitations.16
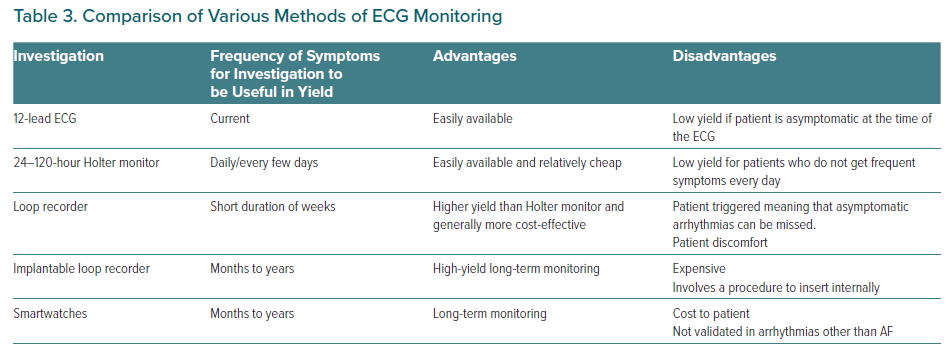
Ambulatory monitoring is available in five formats:
- Holter monitors that are attached to patients for a limited period of a few days.
- External loop recorders and event recorders that can be worn for a prolonged period and are triggered by users when palpitations are experienced.
- Implantable loop recorders (ILR) that are placed subcutaneously for prolonged periods of time.
- Newer ambulatory monitoring devices can offer ambulatory monitoring without the wiring required in normal ambulatory monitoring.17
- Emerging technologies, such as smartwatches with ECG monitoring, which can reliably screen for asymptomatic AF.18,19 However, their role in the investigation of other arrhythmias is much less clear. If the smartwatch uses photoplethysmography for detection, then subsequent confirmation with a formal ECG will be required before treatment.20
Table 3 demonstrates the choices in ambulatory ECG monitoring. Ambulatory monitoring has inherent limitations, chiefly that it is not possible to always make a precise diagnosis, especially when using single-lead devices. It may cause difficulty when deciding if the witnessed rhythm is a supraventricular rhythm with aberrant conduction or a ventricular tachycardia. Furthermore, ambulatory monitoring requires the patient to experience a recurrence of symptoms so that the arrhythmia is captured. This can delay the diagnosis and leaves the patient at risk of malignant rhythms.
Rarely, electrophysiology testing can be performed to enable a detailed analysis of the underlying cause of a cardiac arrhythmia as well as its originating site. However, these are only indicated in patients with a high pre-test probability of a serious cardiac arrhythmia.7,21 Electrophysiology studies can correctly identify the type of arrhythmia responsible for palpitations and enables simultaneous ablation in the same session. This is usually used in high-risk patients with significant heart disease and palpitations associated with syncope as the risk of adverse events from untreated events is unacceptably high. This high-risk cohort of patients can be directly referred for electrophysiology studies without ambulatory ECG monitoring.22,23
Special Situations in Cardio-oncology
Palpitations Due to Psychosomatic Disorders
Anxiety and panic disorders are a frequent cause of palpitations.8 These disorders have been implicated in sinus tachycardia and can modulate increased awareness of one’s own heartbeat. Furthermore, it is known that intense emotions can produce arrhythmias secondary to increased adrenergic activation.24,25 Psychosomatic disorders are particularly relevant in cardio-oncology as it is known that patients suffering with cancer have much higher rates of anxiety, depression and panic disorders.26 This may act as a substrate for palpitations in this group. However, it is important to note that both psychosomatic and non-psychosomatic palpitations are likely to exist at higher rates in the cardio-oncology population as compared to the general population. This is because cardio-oncology patients are likely to suffer from higher rates of anxiety and higher rates of cardiac disease secondary to their age and the chemotherapeutic agents used in their treatment.27–29 Furthermore, cardiac arrhythmias and psychosomatic disorders are not mutually exclusive and can frequently coexist.30 It is also important to note that in one study looking at patients with arrhythmogenic disorders, about 66% were previously diagnosed as having psychosomatic palpitations.31 In the oncology population we advise that this diagnosis should only be reached if the patient has a benign history with a normal echocardiogram, a 12-lead ECG and ambulatory monitoring.
Palpitations Due to Chemotherapeutic Agents
Several chemotherapeutic agents have been implicated in causing arrhythmias. This topic has recently seen several reviews and awareness is increasing.27,32,33 This section will briefly cover the most important implicated agents and their respective arrhythmias.
Ibrutinib is a Bruton’s kinase (BTK) inhibitor that is an effective and well-tolerated treatment for a variety of B-cell lymphomas. Ibrutinib has demonstrated excellent results in chronic lymphocytic leukaemia, small lymphocytic lymphoma, mantle cell lymphoma and marginal zone lymphoma.34 Although generally well tolerated, ibrutinib is estimated to cause AF in about 6–16% of patients.35 This frequently occurs a few months into treatment with over 75% of cases reported in the first year. AF can often be a therapy-limiting side-effect and is the most common reason for patients stopping therapy.36 Therefore, it is prudent in patients presenting with palpitations who are on BTK inhibitors to investigate thoroughly for AF with prolonged ambulatory monitoring. Early referral to cardio-oncology is advised as treatment is more complex and requires joint decision-making on the need for rate/rhythm control and choice of anticoagulation. This cohort has increased risks of bleeding and ibrutinib is known to interact with a variety of medications used in the management of AF.37 We advise a rate control strategy in the first instance unless compelling reasons for rhythm control exist. It is advised to use a direct oral anticoagulant (DOAC) as the choice of anticoagulation.38 Cardio-oncology can also facilitate the use of lower doses of DOACs in patients at high risk of bleeding or a reduced dose of BTK inhibitors to reduce the burden of AF in conjunction with oncology and a multidisciplinary team approach.39
Arsenic trioxide is a potent agent used in the treatment of some leukaemias and myelomas. It is classically implicated in QTc prolongation in 26–93% of patients with rare life-threatening ventricular arrhythmias reported.40–42 This effect is largely transient and occurs 1–5 weeks after infusion and resolves about 8 weeks later.43 In this time patients are at risk of the consequences of prolonged QTc, including torsades de points (TdP), fatal ventricular arrhythmias and sudden cardiac death. The severity of the QTc prolongation can be variable and is related to electrolyte abnormalities and other agents known to prolong the QTc.40 Overall, it is estimated that about one-third of patients experience QTc prolongation >60 ms.40 In this cohort of patients cardio-oncology advice should be sought and close monitoring undertaken in the form of baseline and weekly ECG monitoring.44
Management
The management of palpitations in cardio-oncology is predicated on the underlying aetiology – cardiac arrhythmias, structural heart disease, chemotherapy-induced, anxiety disorders or systemic disorders – and its implicated prognosis. If a definitive aetiology is found and a curative therapy exists, such as ablation for supraventricular arrhythmias, this should be the treatment of choice.45 In the vast majority of benign arrhythmias, such as atrial/ventricular premature/delayed beats, it is advisable to undertake lifestyle interventions prior to pharmacological interventions. It is well recognised that several lifestyle choices can reduce the risk of palpitations and arrhythmias, such as a reduced intake of caffeine, alcohol and recreational drugs. Furthermore, it is well recognised that a lack of sleep and lifestyle stress are linked to palpitations. Furthermore, in the absence of a sinister cause of palpitations, reassurance from a clinician can reduce the overall symptom burden. Overall, oncology patients with palpitations benefit from the same preventive measures that are recommended to the general population.46–48
Another consideration is the prognosis of the underlying cancer. In patients with a good prognosis, it would be reasonable to pursue all treatment strategies and investigations, while in patients with a prognosis that is measured in weeks or months, the value that will be added by some treatment strategies, such as anticoagulation for AF, may be questionable. The question of how to treat and investigate a patient with palpitations should always be preceded by the prognostic implications of the underlying cancer.
A referral to cardio-oncology is always warranted in the context of a suspected chemotherapy-induced arrhythmia as cardio-oncologists can help facilitate better care for patients in liaison with oncology and the multidisciplinary team. Cardio-oncologists can facilitate pre-chemotherapy prophylactic antiarrhythmic therapy, or after discussion with oncology can facilitate reduced-dose chemotherapy regimens. AF is the most common cardiac arrhythmias in the cancer patient population (2–16% of patients during treatment) and management requires similar decisions as those made in the non-cancer population with regards to rate and rhythm control.49,50 However, there exists certain nuances in this population that need to be considered in the decision-making process. Anti-thrombotic therapy is particularly challenging because cancer can result in both a pro-thrombotic and pro-haemorrhagic state and an unpredictable anticoagulation response.51 This is highly dependent on the cancer type as the differential risks conferred by various cancers can be vastly different. Furthermore, the normal risk scores that can be used, such as CHA2DS2-VASc (congestive heart failure or left ventricular dysfunction, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled] – vascular disease, age 65–74 years, sex [female]) and HAS-BLED (hypertension, abnormal renal/liver function [1 point each], stroke, bleeding history or predisposition, labile INR, elderly [>65 years], drugs/alcohol concomitantly [1 point each]) have not been validated in the cancer population. Therefore, in the absence of evidence, an individualised approach is suggested and the decision should not purely hinge on risk assessment scores.52,53
Once a decision to anticoagulate is made, the choice of agent is more nuanced than in the general population. Treatment with warfarin is more complex as efficacy and safety is dependent on the international normalised ratio (INR) and this can be subject to interactions with chemotherapeutic agents. Low molecular weight heparins (LMWH) are often used in the treatment of thromboembolism in this patient group. However, their long-term usefulness in AF is limited by the inconvenience of their need to be injected once a day. DOACs are becoming the de facto treatment of AF in the non-cancer population.20 The evidence base is more scarce in the cancer population. This is because many pivotal trials excluded patients with limited life expectancies and the evidence base is derived from observational studies. In a landmark study by Shah et al. of over 16,000 patients with AF and cancer, DOAC users experienced lower or similar rates of bleeding and stroke compared to those on warfarin and a lower rate of incident venous thromboembolism.54 An expert position paper by the Spanish cardio-oncology society recommends that DOACs should be used as a first-line choice in the treatment of AF in the cancer population.50
AF catheter ablation may be used in a high selective population where a rate/rhythm control strategy has failed and the patient has a high burden of symptoms and/or the chemotherapeutic agents used have a high likelihood of interaction with anticoagulation. There are currently very limited data on the safety and efficacy profile of catheter ablation of AF in the cancer population; however, what limited data exist suggests a higher propensity for bleeding complications.55
A prolonged QTc is more commonly seen in the cancer population due to multiple risk factors including but not limited to advancing age, electrolyte abnormalities and chemotherapeutic agents. A prolonged QTc can lead to TdP, which may be experienced as palpitations by the patient. The duration of the QTc should be controlled before, during and after cancer treatment. A full list of QT-prolonging drugs can be found at Credible Meds (www.crediblemeds.org). The management of prolonged QTc is dependent on correcting the reversible factors (such as electrolyte abnormalities or QT-prolonging drugs). Cardiologists looking after cancer patients should actively rule out long QTc and TdP as a cause of palpitations.
Conclusion
Palpitations are a frequent cause of morbidity and rarely mortality in the cancer population. Palpitations are one of the most common presenting complaints to healthcare providers and are more common in the cancer population where they are under-recognised and under-treated. Therefore, it is essential for the general cardiologist to recognise the nuances of diagnosing and managing palpitations in the oncology population and to know when to refer to their local cardio-oncologist.