Mitral regurgitation (MR) is one of the most common valvular abnormalities worldwide and the second most common type requiring surgical treatment.1 The heterogeneity of MR has attracted the interest of imagers who have identified the need to implement newer advanced cardiovascular imaging techniques to delineate MV anatomy and understand the underlying pathophysiologic substrate of MR.2 Contemporary minimally invasive repair techniques of the mitral valve (MV) cannot be applied in high-risk patients without the use of intraoperative imaging guidance.3
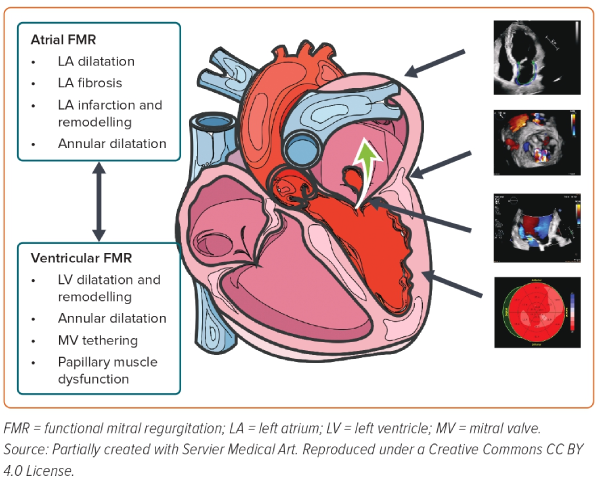
MR is classified as primary MR (PMR) when mitral leaflets and/or subvalvular apparatus are structurally abnormal, and secondary or functional (SMR) when left ventricular (LV) and/or left atrial (LA) dysfunction is present in the absence of organic valvular disease.4 SMR is a heterogeneous valve disease with multiple responsible pathophysiological mechanisms that may complement each other. SMR is usually the consequence of LV or LA dilatation, remodelling, and/or dysfunction leading to coaptation defect due to leaflet tethering, preventing adequate leaflet tip approximation, due to insufficient and maladaptive leaflet tissue growth (Figure 1).4 As a variety of clinical phenotypes of SMR exist, every patient should be assessed thoroughly to clarify the precise pathophysiologic mechanism. Moreover, as SMR is associated with an increased risk of heart failure (HF) hospitalisation and mortality, improvement of SMR severity, long-term prognosis and quality of life is of paramount importance.4,5 Multiple imaging modalities are essential to identify the morphological characteristic findings of the MV, grade MR severity and evaluate total cardiac function so that the optimal therapeutic and interventional approach strategy can be applied to each patient.2
LA function in PMR is an independent prognostic marker and a significant determinant factor in a patient’s clinical status.6 Although PMR is often characterised by LA enlargement and dysfunction, the accurate role of LA function in SMR has yet to be examined. This literature review aims to identify the role of the LA in SMR as a prognostic and therapeutic tool.
We performed a literature search in PubMed and Google Scholar up to May 2023 using the keywords “secondary mitral regurgitation”, “left atrium strain” ([left atrium] AND [strain]) AND (secondary mitral regurgitation). Additional relevant manuscripts detected from the reference list of the articles were retrieved from the initial search.
Normal Left Atrial Function and Speckle Tracking Echocardiography
The LA plays an important role in normal cardiac function as it regulates the cardiac output of the LV by augmenting LV preload, buffers pressure oscillations between the LV and pulmonary vasculature preventing pulmonary congestion and prevents volume overload by regulating the secretion of atrial natriuretic peptide and sodium retention. Thus, the LA acts as a reservoir of oxygenated blood from the pulmonary veins during ventricular systole, a conduit for pulmonary venous return during early ventricular diastole, and a booster pump that increases ventricular filling during the late ventricular diastolic phase, contributing 20% of the total blood volume entering the LV during diastole.7
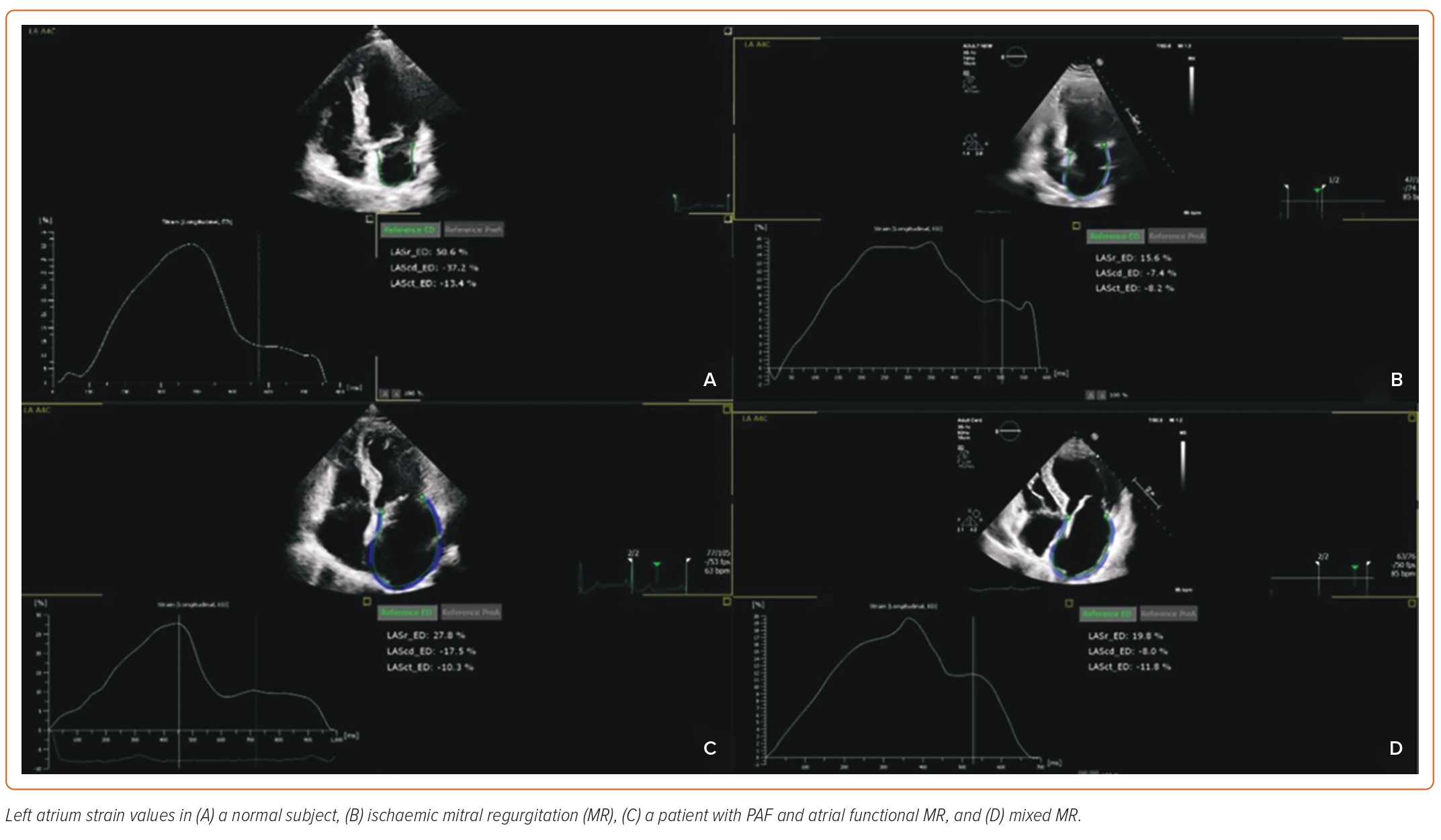
Newer advanced echocardiographic techniques, such as speckle tracking echocardiography, offer accurate recordings of these LA changes throughout the whole LA cyclic phase. Respectively, LA mechanical function can be categorised into three different chronic phases: filling (reservoir) phase; passive emptying (conduit) phase; and active emptying (contraction) phase. Naturally, these three phases are present only in patients with sinus rhythm, as the contraction phase is not present in AF patients. Speckle tracking strain imaging can estimate these chronic phases with the estimation of LA reservoir strain (LARS), LA conduit strain and LA contraction strain, respectively (Figure 2).8
Speckle tracking strain (an evaluation of global and regional deformation) is estimated from the tracking of natural acoustic markers (speckles) generated from the interactions between ultrasound and myocardium. Adequate image quality and relatively high frame rates are needed for accurate speckle tracking.
There are several limitations to LA strain analysis:
First, LA function can be affected by primary LA abnormalities and LV dysfunction; thus, distinguishing between the various responsible components is intricate. In speckle tracking, as LV and LA share the mitral annulus, LA strain measurements may be influenced by LV function and measurements.
Second, there is no gold standard imaging method for LA strain estimation. There are also slight differences in strain estimation and measurements between different vendor software programs. As a result, no standard reference values have been validated.
Third, the far-field location of the atrium, thin atrial walls, and presence of the appendage and pulmonary veins are all factors making LA strain imaging technically more demanding compared to LV strain imaging. High-quality image acquisition and non-foreshortened LA views are essential.7–9
The Role of the Left Atrium in Atrial Secondary Mitral Regurgitation
SMR is further classified into ventricular and atrial MR, depending on which chamber is primarily affected.5 Interestingly, a recent study in Australia showed that almost 40% of SMR cases are due to LA dysfunction and are therefore considered atrial.10
Atrial SMR has been characterised as a novel type of SMR, frequently observed in patients with HF with preserved ejection fraction (HFpEF) and most often, with AF.4 The prevalence of atrial SMR is 3–15% in patients with AF. Although many pathophysiological mechanisms have been correlated with atrial SMR, MV annular dilatation (Carpentier classification type I) is the most prevalent. In AF and HFpEF, significant LA and mitral annulus dilatation contributes to atrial remodelling and consequent loss of leaflet coaptation leading to severe SMR, with a usually normal LV size and function and structurally normal leaflets.11 In addition, insufficient leaflet growth and altered atrial dynamics may contribute to atrial SMR.12 Moreover, patients with AF have impaired function of the mitral annulus characterised by presystolic annular reduction leading to deterioration of the MR. Of note, a recent study showed that sinus rhythm restoration leads to a gradual recovery of presystolic annular dynamics, which in turn decreases atrial SMR severity by improving mitral annular leaflet coaptation.13
The use of advanced 2D echocardiography accompanied by 3D echocardiography offers important anatomic information regarding the geometric morphology of mitral annulus and subvalvular apparatus, such as the existence or absence of leaflet tethering with calculation of ratio of annulus area to leaflet area.14
LA plays an important role in HF and recent studies have confirmed reduced LA function in patients with SMR. LA function assessment with estimation of LARS and LA reservoir work (LARW) was more significant compared to size measurement only.15 Inciardi et al. showed that impaired LA function parameters appear earlier in SMR compared to LA size increase.16 In addition, LARS may also be more indicative of LA fibrosis than LA size. Atrial enlargement is the final expression of LA structural remodelling with myocardial fibrosis being the first and hallmark of the latter. Cardiac magnetic resonance with late-gadolinium enhancement (CMR-LGE) is the gold standard in imaging fibrosis. However, as an inverse correlation between peak LA strain and amount of LA fibrosis and LA compliance has been reported, speckle tracking analysis can also offer further information regarding LA myocardial deformation and contribute to early estimation of a patient’s prognosis.17
LA dilatation has recently been added as a new mechanism causing SMR associated with poor prognosis in patients with HF and reduced LV ejection fraction. Although LV systolic and diastolic function play a fundamental prognostic role in patients with SMR, in a large meta-analysis LA enlargement was demonstrated to be a strong predictor of clinical outcomes.18 The role of LA in heart failure with reduced ejection fraction (HFrEF) has long been reported, yet its precise pathophysiological role is still under investigation. Triposkiadis et al. described multiple pathophysiological mechanisms concerning LA dysfunction in HFrEF.19 In patients with HFrEF, LA dilatation and consequent impairment caused by progressive ventricular dysfunction have been identified as a significant contributor to HFrEF decompensation. Thus, the role of echocardiography in patients with SMR and HFrEF is of great importance, providing information not only on LA dimensions and volume but also on LA function with the use of speckle tracking echocardiography and LA strain indices. These novel echocardiographic parameters have been estimated in different clinical scenarios of SMR patients in clinical practice. Supplementary Table 1 demonstrates recently published papers on this issue.
In contrast to primary SMR, patients with ventricular SMR usually have depressed LV function resulting in increased LV filling pressures, impaired annular contraction and high LA pressure.12 LA size reflects the chronic effects of LV filling pressures, whereas LARS more accurately reflects dynamic LV filling pressures.20 LV filling pressures play a fundamental role in prognosis. Impaired LARS in severe MR is associated with an increased risk of all-cause mortality and LARS estimation contributes to risk stratification in severe ventricular SMR.21 Speckle tracking imaging of LA function may provide useful information regarding LV filling pressures as basic echocardiographic estimation of LA and LARS measurement are strongly correlated with LV end diastolic pressure estimated by haemodynamic measurements.22
Apart from the reservoir phase, there is scarce literature focusing on the conduit LA phase, particularly in cases with preserved LV. For example, Kakiouzi et al. identified LA conduit strain as the strongest predictor of total prognosis in CKD patients on dialysis.23
Left Atrial Strain in Prognosis Estimation of Patients with Atrial Secondary Mitral Regurgitation
Few studies demonstrate the importance of LA strain as a major prognostic parameter in SMR. LARS was used as a mortality predictor in a median 5-year follow-up of 666 patients in a study conducted by Stassen et al., which showed that LARS had an incremental prognostic value over LA volume and LV global longitudinal strain.21 Mesi et al. showed reduced LA strain and worse prognosis in patients with atrial SMR compared to PMR.24
In the future, LA ischaemia and infarction might also be a diagnostic and therapeutic target in patients with HF. An interesting experimental study by Aguero et al. in 2017 showed that extensive scar and fibrosis of the LA myocardium caused by LA infarction as a result of occlusion of the LA branch, not only provoked LA dilation and impairment of reservoir and booster pump functions, but also led to early appearance of ischaemic MR.25 Considering all of the above, LA infarction complicating MI induces LA remodelling and dysfunction. LA strain applications could potentially facilitate the investigation of the underlying pathophysiological mechanisms.
Role of the Left Atrium in Ventricular Secondary Mitral Regurgitation
SMR is also common in patients with HFrEF. Almost 65% of patients with HFrEF and moderate, severe MR are classified as ventricular SMR.1,26
LV dilation and remodelling, observed in slightly more than 55% of patients with dilated cardiomyopathy and regional myocardial wall motion abnormalities with leaflet tethering, affecting almost 35% of patients with ischaemic cardiomyopathy, are the main pathophysiological mechanisms of SMR.27 In these cases, SMR is called ventricular SMR. According to Carpentier classification, the mechanisms of ventricular SMR can be categorised into two groups: type I, where the MV leaflets are normal but the mitral annular dilatation (either due to LA or LV dilatation) leads to leaflet coaptation defect creating a central MR jet (i.e. in dilated cardiomyopathy (DCM)); and type IIIb, where restricted motion of one of the MV leaflets, most commonly the posterior, due to regional wall motion abnormalities or LV dilatation leads to leaflet malcoaptation and consequent MR as appears in ischaemic heart disease (IHD).
Multiple Hits of Ischaemic Heart Disease
There are several different mechanisms responsible for ventricular SMR in the case of IHD. Increased leaflet tethering and consequent coaptation defect led to ventricular SMR. Wall motion abnormalities, LV dilation, remodelling and dyssynchrony along with papillary muscle apical displacement and reduction of LV contractility are common, mainly in anterior-apical infarction.28 Moreover, inferolateral infarction affecting mainly the posteromedial muscle consists of a different phenotype of ischaemic MR, with the MR jet being usually eccentric and directed along the tethered leaflet.29
As previously described, in cases of SMR due to IHD, both the LV and papillary muscles suffer. Regarding leaflet structure and function, recent data focusing on mitral leaflet remodelling showed leaflet enlargement as a compensatory mechanism in dilated LV to prevent the deterioration of MR.30,31 However, this favourable mechanism acts in a deficient way in patients with ischaemic MR.32 In addition, leaflet thickening, extracellular matrix remodelling with increased levels of activated myofibroblasts and transforming growth factor (TGF)-b, as well as increased microvessel formation are among the compensatory mechanisms of MV leaflets in ventricular SMR due to IHD.33–35
Studies focusing on LV remodelling in patients with severe aortic regurgitation (AR) proved the existence of compensatory developing mechanisms of MV leaflets to prevent the appearance of MR.36 An experimental study by Marsit et al. showed that in two groups with AR, the presence of apical MI in one group was associated with MR, while the group with isolated AR exhibited no MR. The investigators supported that the whole cardiac function alters after MI, and several neurohormonal and inflammatory processes are activated, leading to MV thickening, increased expression of extracellular matrix genes, and insufficient leaflet enlargement. In contrast, adequate leaflet enlargement prevents the appearance of MR in cases with severe LV dilatation induced by aortic regurgitation.31 Consequently, the role of Carpentier MR classification is ambiguous in identifying MR mechanism.
Proportionality and Disproportionality of Secondary Mitral Regurgitation
As more complicated and severe SMR cases are quantified in more insufficiently investigated areas, the terms of proportionality and disproportionality appear in an attempt to explain the conflicting results of the MITRA-FR and COAPT trials.
Both studies included patients with significant SMR and HF undergoing transcatheter edge-to-edge repair (TEER), compared to a medically treated control group. The MITRA-FR trial showed that there was no difference in LV end diastolic volume (LVEDV) and clinical outcomes between the two groups, while COAPT showed that patients undergoing TEER had lower mortality and hospitalisation rates, and LV volumes compared to the control group.37,38
In an attempt to work out why there was such a discordance, TEER patients enrolled in these trials were compared, and interestingly, it was found that in the COAPT patients, the effective regurgitant orifice area (EROA) was approximately 30% higher but LV volumes were approximately 30% smaller. These findings led to the perception of proportionately and disproportionately severe MR. In other words, patients with proportionate SMR, relevant to the degree of LV dilatation, did not seem to benefit from TEER, whereas those with SMR which did not relate to the LV volume, had more favourable results after TEER.37,39
Thus, apart from the distinction between PMR and SMR, proportionality status should be considered when assessing the severity of SMR as it may help in clinical decision-making.
Left Atrial Strain in Prognosis Estimation of Patients with Ventricular Secondary Mitral Regurgitation
LA function in patients with reduced ejection fraction and ventricular SMR should always be evaluated in clinical practice. Malagoli et al. showed that among patients with non-severe SMR, only those with reduced LARS had worse prognosis. An estimated LARS value lower than 14% had a negative prognostic effect over an almost 4-year follow-up in patients with ventricular SMR.40 Furthermore, a recent study by Gomes et al. that included patients with reduced EF and at least mild ventricular SMR demonstrated that LARS is independently associated with all-cause mortality with a cut-off value of 15%.41 Besides, the LA dynamic profile was found to be impaired in patients with SMR as lower LA strain and strain rate values were demonstrated in MR patients both at rest and at exercise associated with worse clinical outcomes.42
Left Atrial Function in the Treatment of Secondary Mitral Regurgitation
Cardiac Resynchronisation Therapy and Left Atrial Function in Secondary Mitral Regurgitation
Cardiac resynchronisation therapy (CRT) is a recent therapeutic technique used in the management of patients with HFrEF, when LV dyssynchrony and severe ventricular SMR with persistent symptoms exist despite optimal medical therapy. In patients with LV dyssynchrony, CRT has been shown to improve LV function and reduce or eliminate MR in some patients, while cessation of CRT function results in the reappearance of MR, which in turn, is ameliorated by re-initiation of CRT function.
In patients with HF and severe ventricular SMR, LA afterload is also increased by the elevation of LV end diastolic pressures, while LA volume overload leads to LA adverse remodelling. Limited data do exist regarding the effect of resynchronisation therapy on SMR and LA function. Previous studies have demonstrated that SMR severity is independently associated with LARS, and LARS in turn is associated with all-cause mortality and provides incremental prognostic value over LA volume.43–45 Stassen et al. recently investigated the relationship between LA function and SMR before and after CRT implantation. Interestingly, they noted that there was a significant decline in MR severity at 6 months after CRT implantation, which in turn was independently associated with an increase in LARS. Furthermore, an increase in LARS was independently associated with lower all-cause mortality in patients with HF and significant MR after CRT implantation, leading to the conclusion that the improvement in MR severity was associated with better prognosis.46
More specifically, patients with improved MR severity and concomitant increase in LARS value had the lowest mortality rates. On the other hand, there were no significant differences between patients whose MR was improved but with no increase in LARS value, and MR non-improvers. Thus, an impaired LA dynamic profile reflects abnormal LV filling pressures and is influenced by the hemodynamic changes provoked by the underlying SMR. CRT treatment may partially restore impaired LA, leading to better prognosis. It is worth noting that LARS was the only evaluated echocardiographic parameter that showed improvement after CRT and an increase in its value could be indicative of LA reverse remodelling.
Left Atrial Function and Transcatheter Edge-to-Edge Repair of Secondary Mitral Regurgitation
Transcatheter edge-to-edge repair (TEER) has been established as a minimally invasive procedure for treating secondary MV regurgitation.3 Recently, two randomised controlled trials (RCTs), MITRA-FR and COAPT, assessed the efficacy and safety of the MitraClip in patients with systolic HF and severe ventricular SMR.38,47 Due to the opposing results of the trials, with the larger trial showing significantly positive results and the other trial showing negative results, these trials challenge investigators to find out which patients benefit from TEER and which do not, as both trials included a very broad spectrum of SMR patient population.
SMR patients with increased LVEDV consequently have a larger effective regurgitant orifice area (EROA) and regurgitant volume (RVol). On this concept, patients with disproportionately severe SMR compared to the degree of LV dilatation benefited more from TEER in RCTs of MV repair (MITRA-FR and COAPT trials).39
TEER has been shown to have a more beneficial effect in patients with severe MR but no severe LV dilation.48,49 However, Messika-Zeitoun et al., in their sub-study (MITRA-FR), showed no effect of TEER in severe SMR independently of the EROA, RVoL, LVEDV and LV ejection fraction estimation.
TEER is an effective treatment strategy not only for ventricular SMR but also for atrial SMR with high procedural success rates, significant clinical improvement and improved survival rates.50 Sudo et al. reported that percutaneous MV intervention (TEER or transcatheter MV replacement [TMVR]) decreases HF hospitalisations and improves NYHA class functional status more effectively compared to medical treatment alone.51
Moreover, optimal medical treatment uptitration after TEER is associated independently with lower mortality and hospitalisation rates and higher MR decrease at 6-month follow-up.52
It is worth noting that the evaluation of LA functional parameters, such as LARS estimation, and their association with SMR after TEER, have not yet been widely investigated. TEER has been shown to increase LA strain value with decreased LA stiffness in five studies conducted since 2016. Toprak et al. demonstrated improved LA reservoir and LA contraction strain within 12 months after successful TEER of the MV, lower LARS value and higher 3D LA volume pre-procedurally were associated with worse prognosis.53 Moreover, Ro et al. described reduced LA conduit strain combined with decreased LV volumes in patients undergoing MitraClip, 1 month after the procedure.54
A small study conducted by Scislo et al. in patients undergoing TEER of the MV surprisingly showed no improvement in myocardial strains (LV, LA, RV) but deterioration of LARS in some subgroups.55 Another study showed LA strain decrease after TEER but included only a small number of patients (25% of whom had SMR).56 Finally, Ozturk et al. showed that baseline atrial global strain (aGS) was the strongest predictor of mortality and adverse outcomes after MitraClip; however, aGS exhibited no significant changes during follow-up.57
The possible association between LA function improvement after transcatheter MV repair with improved prognosis needs further investigation.
Clinical Implications and Future Perspectives
Current European guidelines suggest the treatment of severe SMR after receiving optimal medical treatment and CRT in patients with persistent symptoms, either with surgery (class I if undergoing other cardiac surgery; class IIb otherwise) or TEER (class IIa).3
While PMR guidelines include clinical (new onset of AF) or echocardiographic (severe LA dilatation) parameters to proceed to surgical treatment, these parameters have not yet been implemented in SMR, in order to help the decision about optimal timing for intervention.
3D echocardiography, exercise echocardiography and CMR may be used to identify patients with severe MR. Dynamic 3D MV modelling evaluation as well as new parameters, such as mitral leaflet coaptation reserve should also be examined. However, LA parameters measured in these modalities have not yet been thoroughly investigated.
Until now, there have been no specific medical therapies for restoration of LA remodelling and function. Recently, a risk model has been introduced to predict outcomes after surgery in PMR patients.51 Maybe it is time to let artificial intelligence guide us in decision-making for SMR intervention by using an equivalent model that will take several clinical and echocardiographic factors into account.
Conclusion
SMR includes a variety of complex pathophysiological mechanisms, and it is essential to evaluate the haemodynamic and morphological profile of the whole cardiac function. Current guidelines have conflicting indications regarding the evaluation of severity and the timing of intervention. LA function plays a major role in the diagnosis, therapy, and prognosis of MR patients. However, whether LA function evaluation should be the future therapeutic target needs further investigation.