The impact of CT coronary angiography (CTCA) within diagnostic cardiology has been far-reaching and profound. The clinical utility of this modality is underpinned by excellent sensitivity (99%) and negative predicative value (97%) for detecting significant coronary artery disease.1 Over the past decade, technological advances in the form of increased gantry spin times and fast single heart beat scanning have driven improved temporal resolution together with lower radiation exposure. These advances have been realised through prospective ‘gating’ of the CT study. Coronary arteries are mobile throughout the cardiac cycle, and imaging during the phases where they are least mobile is paramount to image quality. This occurs typically at 50–80% of the R-R interval. The X-ray tube is only active for a specific duration of the cardiac cycle; for example, 50–80% of the R-R interval, thus reducing radiation exposure.2
Increased gantry spin times and multidetector technology have allowed the development of specific algorithms that allow whole heart scanning in a single heartbeat. For example, the time required for one rotation of an X-ray tube is reduced by increasing the gantry spin times and effectively halved by adding a second X-ray tube. This allows acquisition of the whole heart volume in a single beat at a specific point of the R-R interval. The patient is then moved rapidly through the z-axis to cover the whole heart in a single acquisition.
In addition, vendors offer models with an increased number of detector rows (256 and 320 slice), which allows coverage of the entire heart in a single acquisition. The advantages of these techniques are that the X-ray tube is only on for a small amount of time and it alleviates stitching artefacts, as the whole image is acquired in one sequence. All the major CT vendors have recently introduced systems incorporating these advances. Rigorous heart rate control is also essential for keeping the effective radiation dose down. The effective dose given to an average patient doubles when their heart rate increases from <55 to 60 BPM. It doubles again with an increase to 65 BPM.3
The culmination of recent advances led to a change in the National Institute for Health and Care Excellence (NICE) guidelines for assessment of chest pain of recent onset.2 CTCA is now recommended as the first-line investigation for patients with typical or atypical chest pain. This represents a dramatic change from previous versions of the guidance, with a move away from calculations of pretest probability and the previous emphasis on functional imaging. The radiological infrastructure within the UK is significantly behind other European health systems, and the change in NICE guidance will contribute significantly to increased demand. Significant investment, in both infrastructure and personnel, will be required over the next decade, and this will remain a challenge within the UK for many years to come. The British Society of Cardiac Imaging produced a report on the provision of cardiac CT that estimated a UK-wide shortfall of 43%.4
The importance of CTCA as the principle non-invasive imaging modality in stable chest pain patients is highlighted in the 5-year outcomes from the Scottish Computed Tomography of the Heart (SCOT-HEART) study. When added to standard care, CTCA was associated with fewer cardiovascular disease (CVD) deaths or non-fatal MIs (HR 0.59; 95% CI [0.41–0.84]; p=0.004).5
In this review, we analyse the recent advances in CTCA, including how coronary artery calcium scoring (CACS) is being implemented to risk stratification algorithms; the development and refinement of CT-derived fractional flow reserve (FFR); plaque morphology and plaque characteristics, and how these may effect prognosis; and the development of machine learning (ML), and how these advances may impact clinical practice in the coming years.
Coronary Artery Calcium Score: Precision Medicine and Risk Modification
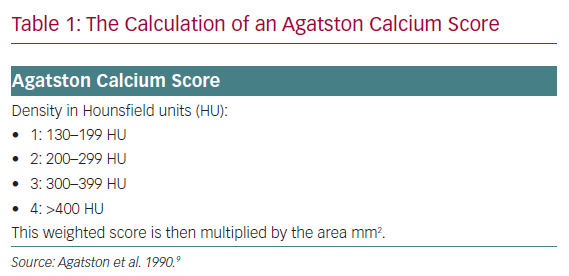
The pathological evolution of atherosclerosis is a dynamic process involving varying inflammatory insults to the arterial intima, which ultimately results in the development of coronary plaque.6 The evolution of atherosclerosis is highly variable, and there is a spectrum of atherosclerotic disease with distinct plaque characteristics. One common final pathway for plaque progression is calcification.7 Coronary artery calcification is associated with total atherosclerotic burden and advancing disease phenotypes.8 There are several methods used to quantify the burden of calcification, including the Agatston score, calcium volume and, more recently, calcium density. The Agatston score is the most established of these methods due to its good reproducibility and high accuracy. The Agatston calcium score was initially developed in the 1990s as a tool for quantifying the degree of calcium within the coronary arteries. Calcification within the coronary artery is defined as 130 HU and >1 mm2 in size. The method for calculating the Agatston score relies on calcium density and total area of calcification (Table 1). Early work was performed using single-beam electron beam CT, and later expanded to multidetector CT.9
Several large cohort studies have established CACS as a valuable tool to assess future CVD risk.10–12 The outcome of CACS is traditionally categorised into different strata reflecting the differing CVD risk. Unsurprisingly, the lowest risk of any coronary event is in the CACS 1–100 strata (HR 3.61), and the highest in those >300 (HR 9.67).10 Although the CACS technique has been in use for nearly two decades, there have been recent advances incorporating its use in regard to risk stratifying individuals. Unsurprisingly, it provides incremental benefit above traditional CVD risk prediction models, and is underpinned by the ability to potentially reclassify patients assessed by traditional CVD risk scores.13–17
Changes in risk prediction algorithms have traditionally been assessed by changes in the area under the receiver operating characteristic curve, but a more recent statistical method, the net reclassification index, is being preferentially used. The net reclassification index is the extent to which people are appropriately reclassified into higher or lower risk categories, and thus serves as a quantitative measure for the performance of risk prediction models when a new marker is added.18 The ability to reclassify patients is an important concept, as it allows increased precision of primary prevention medication prescribing and targeted risk reduction strategies. In addition to targeted medication, CACS remains a powerful mediator for lifestyle change.19
Recently, CACS has been added to traditional risk factors to create a risk prediction tool. The Astronaut Cardiovascular And Health Modification (Astro-CHARM) tool measures 10-year atherosclerotic CVD risk, and was developed using three large cohorts and validated against a fourth.20 The Astro-CHARM calculator again demonstrated the incremental value of added CACS above the Framingham Risk Score (improvement in C-statistic of 0.03 and net classification improvement of 0.12). A previous CVD risk calculator developed using Multi-Ethnic Study of Atherosclerosis (MESA) data was validated in an older cohort (mean age 65 years in MESA and mean age 51 years in Astro-CHARM).21 Statins are indicated in patients aged 50 years with a CVD risk profile >7.5–10%.22,23 Given that age is a significant independent CVD risk factor, the Astro-CHARM tool has potentially greater clinical utility.24
The development of integrated risk prediction tools enables enhanced decision-making around primary prevention medications and risk factor modification. The current European guidelines give CACS a level IIa recommendation for intermediate risk patients.23 In a review of the current evidence, Greenland et al. made specific recommendations regarding the CVD risk in the context of CACS. Essentially, if the 10-year risk of a CVD event is 7.5–20%, but the CACS is zero, then statin therapy may not be required due to the lack of impact on events.25
The scope for CACS ± CTCA to be used in high-risk patient groups in which the Framingham Risk Score may underestimate the true CVD risk is increasing. For example, CVD risk calculators in HIV (and other chronic inflammatory diseases) are known to underestimate true CVD risk.26 Patients with HIV have been shown to have greater CACS than matched non-HIV patients, illustrating the greater burden of subclinical coronary atherosclerosis.27 There are currently no validated risk predication tools that incorporate CACS in these higher-risk populations.
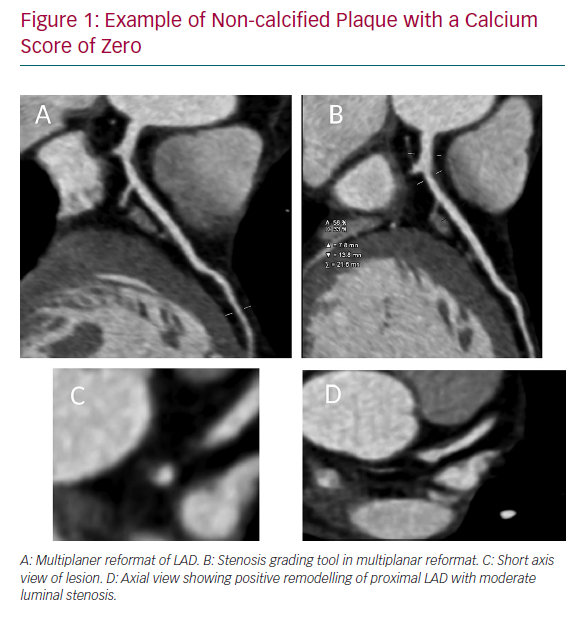
Recently data from the PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) cohort have been reported around the prognostic benefit of a CACS of zero in patients (n=8,811) symptomatic of chest pain. Evaluating the performance of CACS to functional imaging, the investigators found that in symptomatic patients with CACS of zero the annualised event rate was <1%. Out of 133 events, there were 21 patients who had a CACS of zero (15.8%), and of those patients, only two had a severe non-calcified (>70%) stenosis. The risk of having an event with a severe non-calcified stenosis (>70%) with a CACS of zero was 1.4%. Of these 21 patients, approximately half had normal coronary arteries (52%), thus the event may actually have been secondary to a type 2 MI, embolism or coronary spasm (Figure 1). There were no data on plaque morphology. Having a positive CACS (≥1) was able to predict 83% of future CVD events, whereas a positive functional test only predicted 33%.28 These data add to historical evidence that CACS of zero is associated with a very low risk of CVD outcomes.29
CT Coronary Angiography and Functional Assessment
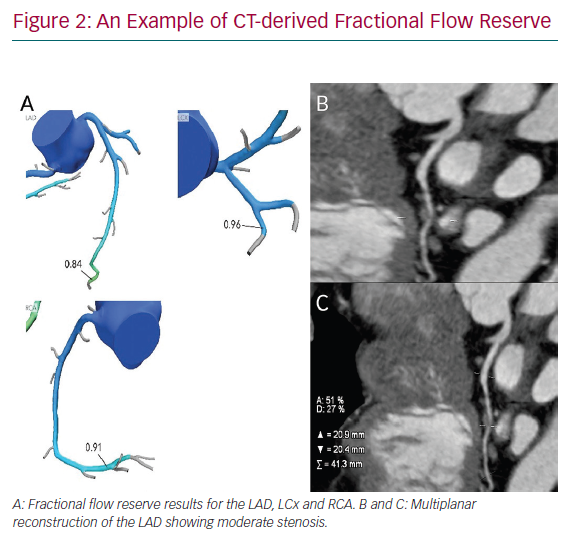
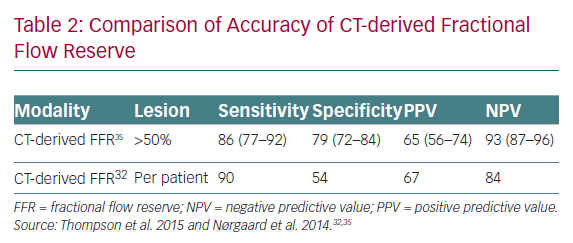
Fractional flow reserve CT (CT-derived FFR) uses computational fluid dynamics to predict the functional significance of coronary artery lesions. In the current and only commercially available model, data from standard CTCA data sets are transferred to the vendor’s server with a report emailed back within a few hours. The report demonstrates the coronary tree with CT-derived FFR results reported in all coronary segments (Figure 2). In a similar fashion to invasive FFR, <0.8 is considered functionally significant. The ability to combine both an anatomical and functional test is extremely appealing, and this technology is well validated in terms of accuracy and safety (Table 2).30–33 Invasive FFR is a well-established technique for quantifying lesion-specific ischaemia, and is comparable to functional imaging.34 To date, no trials with large numbers of patients have presented a comparison of myocardial functional imaging versus CT-derived FFR.
The technique has several constraints. First, good image quality is paramount. A recent series of CT-derived FFR reported the rate of unsuitable studies as high as 13%.35 In clinical practice, the rate of unsuitable data sets may be higher secondary to inappropriate heart rates (including AF), patients body habitus, poor contrast evolution and problems with artefacts (such as breathing and movement artefacts). Patient preparation and heart rate control has to be rigorous to obtain quality images to use CT-derived FFR. The advent of modern CT scanners will also enhance quality. Second, concerns remain around the diagnostic accuracy in the “FFR grey zone” – CT-derived FFR 0.7–0.8. This was reported as 46.1% in a recent systematic review assessing 536 patients from five studies.36 Third, there are no data for patients having already undergone revascularisation.
Currently, this service is limited to a few dedicated centres in the UK from a single vendor, HeartFlow. NICE have recently updated their guidance to suggest how CT-derived FFR may be considered for patients with recent-onset or stable chest pain.37
As this technology continues to mature, the initial results from the Assessing Diagnostic Value of Non-invasive FFRCT in Coronary Care (ADVANCE) Registry unsurprisingly show that the strongest predictor of positive CT-derived FFR is stenosis >70%.38 Invasive studies of FFR have proven there is a disconnect between anatomical assessment of coronary stenosis and the physiological impact of those lesions.39 The ADVANCE Registry again demonstrates this disconnect, as despite stenosis >70% being the greatest predictor of CT-derived FFR <0.8, there was nearly one-third of severe lesions (28.4%) that were functionally insignificant. Similarly, in patients with non-obstructive coronary anatomy (stenosis grading 30–49%), there was a positive CT-derived FFR rate of 20.8%.
Very recently, the 1-year data from the ADVANCE Registry was published, demonstrating low rates of MI in CT-derived FFR >0.8 (0.19% of total FFR >0.8 group). In addition, 92.9% of individuals in which medical therapy was recommended remained free from revascularisation or major adverse cardiac events at 1 year.40
As the CT-derived FFR technology is refined over the coming years, it is expected to become increasingly available across the National Health Service. The economic impact of this has been considered by NICE when compared with invasive angiography. Conservative estimates show this test could be used in approximately 40,000 patients per year with savings per year of £9.1 million from reduced referral for functional investigation or invasive investigation.37 CT-derived FFR is certain to have an increasing presence in the diagnostic armoury of the cardiologist in the near future, particularly as it offers results on anatomy and physiology in a single test/single visit.
As with all non-invasive cardiac imaging, patient selection will be paramount in delivering this service. In addition, centres will require robust systems for optimising CTCA data set acquisition to ensure quality images for CT-derived FFR analysis.
Plaque Morphology Quantification
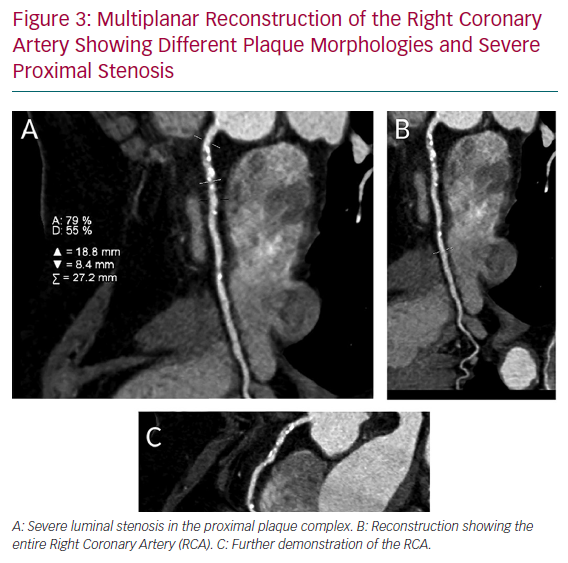
The analysis of plaque morphology is becoming increasingly important. The ability of CTCA to visualise the entire vessel has several advantages in terms of assessing plaque. There has been extensive recent work on how plaque morphology may impact primary prevention, predictors of ischaemia and prognosis (Figure 3).
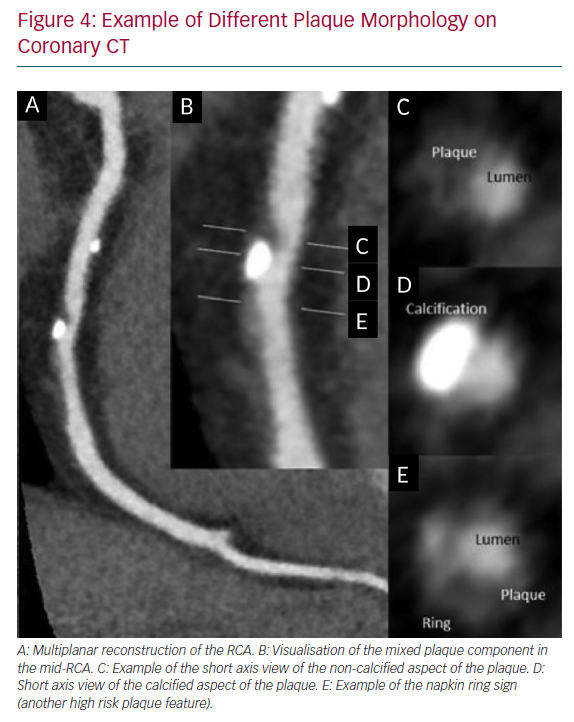
The seminal work by Motoyama et al. established the CT-based concept of vulnerable plaque (VP) identification on CT, which confers a significantly heightened risk of acute coronary syndrome.41 They assessed 3,158 patients for three high-risk features that are readily identifiable on routine CTCA: low-attenuation plaque (<30 HU), positive remodelling of the coronary vessel (remodelling index of >1.1) and spotty calcification. The event rate was 16% over a median follow-up period of 3.9 years.41 High-risk plaque found on CTCA, specifically the plaque composition (necrotic core/fibrous plaque ratio) correlates with thin-cap fibroatheroma, which is seen on intravascular ultrasound.42 Positive remodelling and low-attenuation plaque have also been demonstrated to be associated with thin-cap fibroatheroma with macrophage infiltration on optical coherence tomography (Figure 4).43
Further work around vulnerable plaque in people with type 2 diabetes has recently been published that challenges the high-risk nature of vulnerable plaque morphology. In a series by Halon et al. that included 630 patients, it was shown that VP caused acute coronary syndrome in 3.5% of instances over a median follow-up period of 9.2 years compared with 0.6% of other plaques.44 In other words, 96.5% of VP never causes an event, and the risk attributable to VP was the same as stenosis >50%. The rate of statin therapy was similar at baseline and follow up between ACS cases and non-ACS cases (75–80%). Although there are important differences in the demographics between these two studies, some of the difference in the event rate attributable to VP could be due to baseline statin use. In the Motoyama et al. cohort, the statin rate after initial CTCA was 38.9% compared with 80% in the Halon et al. cohort.41,44
Despite some disagreement on the exact magnitude of the risk conferred by VP, the detection of vulnerable plaque on CTCA is frequently regarded as an indication for aggressive primary prevention strategies. While statins are considered to help reduce the size of plaque and increase calcification, there does not seem to be major evidence that treating those patients with a CACS of zero confers any benefit in terms of hard cardiovascular endpoints. In a cohort of 13,644 patients, statin therapy was shown to reduce the CVD endpoints in patients with evidence of any coronary calcification (adjusted subhazard ratio 0.76), whereas in the CACS of zero group, there was no risk reduction (subhazard ratio 1).45 We do not yet know the impact of treating only non-calcified plaque with aspirin and statin therapies; however, this seems the obvious course to take.
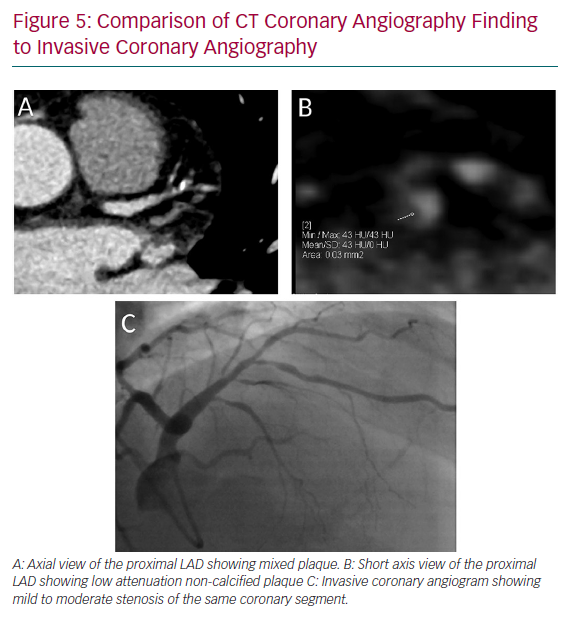
Beyond VP, there are a number of markers obtained on routine CTCA data sets that are used to attribute risk. There have been recent calls to standardise the reporting of CTCA and move away from a qualitative approach that is mostly employed clinically. This is outlined in the Coronary Artery Disease – Reporting and Data System document, and may involve using semi-automated scoring systems alongside traditional luminal stenosis.46 Diameter stenosis, specifically obstructive coronary artery disease, is still the most clinically relevant tool that prompts referral for invasive assessment, and is the marker associated with adverse outcome (Figure 5).47 Other markers, such as segmental stenosis score and segmental involvement score, are used to quantify the burden of disease and both show prognostic value.48,49
The addition of individual plaque characteristics to diameter stenosis can improve the prediction of functionally significant lesions. Sophisticated semi-automated systems are used to quantify plaque characteristics, such as AutoPlaque (Object Research Systems). These systems are limited to research tools and currently have limited scope in the clinical domain, but may well enter routine clinical practice in the foreseeable future.
This disconnect between anatomical assessment and functional importance of a lesion is likely multifactorial. First, the grading of a stenosis may be inaccurate. Second, the location of the lesion and the amount of myocardium subtended is likely to have a role. Third, the plaque morphology at the site of stenosis is likely to portray underlying endothelial dysfunction. The metabolically active plaque confers more endothelial dysfunction, which may contribute to ischaemia secondary to reduced nitric oxide bioavailability.50 To this end, Doris et al. recently published an analysis of CT-derived FFR in non-severe lesions on CTCA. They demonstrated that the best predicator of total vessel ischaemia was total plaque volume (OR 2.09) compared with calcified plaque volume, non-calcified plaque volume and low-density non-calcified plaque volume (OR 1.36, 1.95 and 1.95, respectively).51
Total plaque volume has also been shown to be discriminatory of ischaemia when added to stenosis severity. Øverhus et al. recently published data demonstrating the superiority of total vessel plaque versus proximal plaque in predicating ischaemia. This substudy of the HeartFlow Analysis of Coronary Blood Flow Using Coronary CT Angiography (HFNXT) trial demonstrated improvement of the area under the curve of 0.83 versus 0.81 when whole vessel low-density non-calcified plaque volume was added to diameter stenosis versus proximal plaque. Stenosis severity alone had an area under the curve of 0.78.52 A whole vessel approach is more predicative of overall plaque burden, as it takes into account distal disease.
Total plaque volume across the entire coronary tree is also associated with cardiac-related death. Hell et al. retrospectively assessed 2,748 patients, and found that total plaque volume >179 mm3 was associated with an increased risk of cardiac death (HR 2.3; 95% CI [1.09–4.58]; p=0.022) for over a mean follow-up period of 5.2 years.53 This is unsurprising, as higher plaque volumes portrays more advanced disease phenotypes.
Changes in plaque morphologies over serial CTCAs have the potential to show benefit from primary preventative strategies; the hypothesis being that one would be able to demonstrate to patients reduced plaque volume/VP/stenosis severity in response to specific interventions. Statin therapy is the single most significant pharmacological primary prevention medication, and is well documented to reduce events in secondary prevention cohorts and moderate- to high-risk primary prevention cohorts.54 The influence of statin therapy on coronary plaque has been previously described in intravascular ultrasound virtual histology studies that show reduction in total plaque volumes and increases in dense calcium plaque volumes, although there was no effect on lipid-rich cores.55 Also, the statin effect on coronary plaque is not uniform across agents or dosages.56
On serial CTCA, total plaque volume has consistently been shown to be reduced and calcified plaque volume increased if a significant LDL-lowering target is achieved.57 This progressive calcification in the setting of lipid-lowering therapy is yet to be fully elucidated, but the effect likely represents plaque stabilisation. This should not be confused with the fact that an increasing coronary artery calcification score confers an increasing risk, as discussed. Calcium on CACS is a surrogate for total plaque volume and the extent of potential disease. Increasing calcium arcs within a particular plaque is a sign of a more stable plaque.58,59 These facts call into question the utility of performing serial CACS on any patients taking statin medication without considering density. There are also newer models being developed to try and improve on the basic Agatston score.60
Machine Learning
ML uses computer-based algorithms to make decisions based on multiple variables without having to know the relationship of those variables to the outcome at the outset of the learning period, or even which variables should be included in the predictive model. It has multiple applications in everyday life and is being increasingly applied to clinical imaging. The key ability of ML with diagnostic imaging is to analyse large data sets and extract the applicable data. With CTCA data sets, there is the potential to improve diagnoses and predicating functionally significant lesions. In addition, it will be able to automatically quantify markers, such as calcium scoring, epicardial fat volumes and liver Hounsfield units (to diagnose fatty liver), and plug these data into scoring systems.61 ML and its applications to CTCA has been previously very well reviewed.62
The integration of ML into clinical practice will bring exciting opportunities in terms of risk prediction. In a feasibility study, Motwani et al. analysed 10,030 patients from a large registry with 5-year follow-up data. They analysed 25 clinical and 44 CTCA parameters for predicting risk. ML was significantly better at predicting mortality than any individual clinical or CTCA-based risk factor (area under the curve for ML 0.79, Framingham Risk Score 0.61, segmental stenosis score 0.64 and segmental involvement score 0.64).63 Although that study did not take into account the more sophisticated risk stratification calculators (such as Astro-CHARM or MESA), it demonstrates the potential clinical utility of ML. ML has also been demonstrated to be superior at detection of ischaemic lesions by calculating CT perfusion and adding it to stenosis severity.64 ML-based fractional flow reserve has also been shown to perform well at predicting ischaemic lesions.65,66
The integration of ML into the clinical realm is likely to become reality in the coming decade. As electronic patient records are being increasingly used, the scope for ML is increasing. The potential benefit of ML is multifaceted, and would include enhanced precision of diagnoses and ischaemia, enhanced risk predication (from analysis of countless variables), and reduced healthcare costs from reduced reporting times.
Conclusion
Techniques using CT have improved the landscape of non-invasive diagnostic cardiology significantly over the past decade. The short- and long-term future is set to yield significant leaps forward. CT-derived FFR is highly likely to become increasingly prevalent with the aim of increasing the accuracy of patients requiring invasive assessment/revascularisation. In the subset of patients with previous percutaneous coronary intervention, CT perfusion techniques are currently being investigated. The ADVANCE study is currently recruiting with the aim of reporting the diagnostic accuracy of this technique.67
Potentially, the greatest role of CACS and CTCA is in a primary prevention setting. The addition of a calcium score to traditional risk calculators significantly enhances the accuracy of risk calculators (Astro-CHARM and MESA), which in turn allows a substantial proportion of individuals in the intermediate risk category to be reclassified up or down risk profiles. In addition, the traditional stratification systems for higher-risk populations, such as patients with chronic inflammatory diseases, have been shown to greatly underestimate coronary risk. It may be that CACS takes a more prominent role in achieving enhanced precision in CVD risk estimation in these groups. This will also allow targeted primary prevention strategies in the age of individualised medicine.
There has been much debate very recently regarding a strategy of offering a CACS as a standalone test for the assessment of stable chest pain. The reported prognosis conferred by a calcium score of zero in symptomatic patients varies significantly between cohorts. In their substudy of the Coronary Artery Evaluation Using 64-Row Multidetector CT Angiography (CORE-64) data, Gottlieb et al. demonstrated that 19% of patients with a calcium score of zero had a stenosis >50%. In addition 12.5% of the zero calcium arm went on for revascularisation.68 In contrast, Mittal et al. reported an all-cause mortality of 1.4% in patients with zero calcium, with none of the patients dying of a coronary event. Although 1.7% of the zero CACS group had >50% stenosis, flow-limiting disease was only proven in 0.3%.69 There are important differences between these two studies, not least the differing pretest probabilities and recruitment setting. Other large cohorts have published varying incidences of NCP and outcome data, but we use these two examples to highlight the significant variation. Proponents of a CACS-only diagnostic strategy will highlight the reduced scan time, radiation and contrast risk to patients, and reduced healthcare costs, which are important concepts in the current age. Further research needs to be performed to analyse the diagnostic and prognostic prowess of a CACS-only strategy in stable chest pain. This is especially important given recent UK guideline changes incorporating CTCA as first-line investigation of stable chest pain.
As with all evolving technologies, there are limitations to these emerging technologies. CT-derived FFR is still in its infancy. The applicability of this technique to routine clinical CTCA data set needs to be proven given the rejection rate of up to 13% in registry studies.35 The delivery of modern CT hardware incorporating improved spatial resolution may go some of the way to alleviate this issue. Although the latest ADVANCE 1-year outcomes demonstrate an excellent prognosis in FFR values >0.8, there remains questions to be answered regarding the accuracy with up to 20% positive FFR values in unobstructed coronary arteries. One recent study by Ghekiere et al. compares CT-derived FFR with invasive estimated FFR and stress cardiac magnetic resonance in 37 patients with intermediate lesions. A positive correlation was found with semiquantitative measures of ischaemia on cardiac magnetic resonance and CT-derived FFR (r=−0.63).70 Further fully powered studies comparing CT-derived FFR and other functional modalities will be performed to confirm the accuracy of this technology.
Ultimately, the use of CTCA and CACS is set to show strong growth. As techniques are refined, an ever-increasing scope for precision medicine will come to the fore, and in our view, these benefits will be strongly supported and amplified by the exciting advances in ML.