Stroke is the second most common cause of mortality worldwide and the third most common cause of disability.1 Although there has been a global trend towards a reduction in stroke incidence, prevalence and mortality since the 1990s, the overall stroke burden in terms of absolute number of people affected continues to increase.2 More than 1 million people have a stroke every year in Europe and that figure is estimated to rise to 1.5 million by 2025, due to the ageing population.3
There are three main types of stroke: ischaemic, intracebral and subarachnoid haemorrhage. In the US, the proportion of ischaemic strokes, intracerebral haemorrhage and subarachnoid haemorrhage is 87%, 10% and 3%, respectively.4 These percentages seem to be similar globally, with a trend of a higher increase in the frequency of haemorrhage in developed countries in relation to developing countries, while death rate is significantly higher in developing countries compared with developed countries.5–7
Men have a higher incidence of stroke than women at younger ages, with the incidence reversed by the age of 75 years, although recent data suggests this may not be the case for black people as the stroke risk for black women aged 65 to 74 years was similar when compared with black men.4,8 The finding could be driven by race and sex group differences for stroke risk factors, mainly hypertension.8
Hypertension is the most prevalent risk factor for stroke, based on data from 30 studies, and has been reported in about 64% of patients with stroke.2,9 In low-income countries, the reported prevalence of risk factors among patients with stroke is lower, however patients have the highest in-hospital mortality, probably due to delays in presentation for seeking acute stroke care, differences in health system response and acute stroke management.10
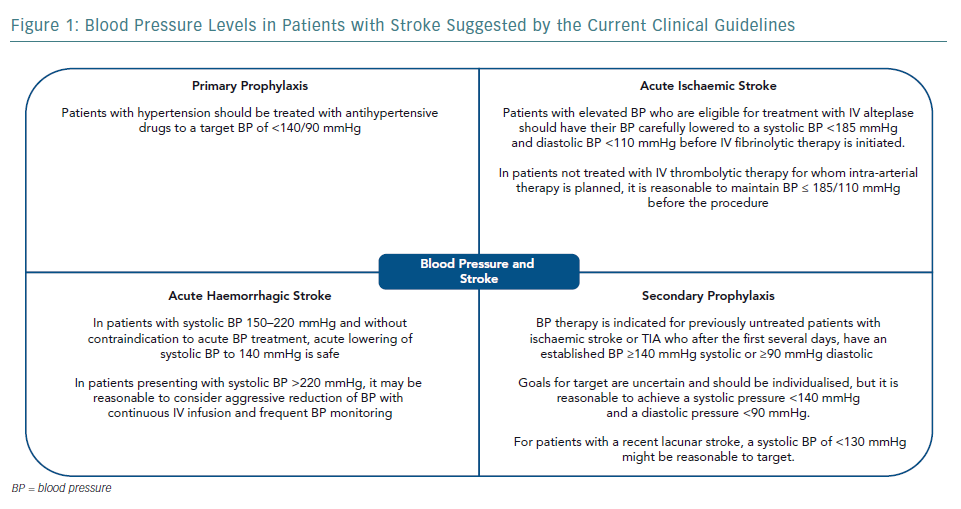
The cause of stroke and haemodynamic consequences are heterogeneous across stroke subtypes and timing of disease presentation. Thus, the management of blood pressure (BP) in stroke patients is complex and requires an accurate diagnosis and precise definition of therapeutic goals. The present review will address the management of BP in patients with stroke, mostly based on recent published guidelines. In general, guideline recommendations from different countries are similar, including the gaps in evidence and suggestions for the need for further studies (Figure 1).11–15
Blood Pressure and Primary Prevention of Cardiovascular Disease and Stroke
There is robust evidence that screening and treatment of hypertension prevents cardiovascular disease (CVD) and reduces mortality in the middle-aged population (50–65 years). Even in older adults, lowering BP is likely to be beneficial provided that treatment is well tolerated, despite a lack of studies to support this. However, there is a lack of high-quality evidence for a favourable harm–benefit balance of antihypertensive treatment among older adults, especially among the oldest age groups (>80 years).16
There has been a debate about how far BP should be lowered. The American Guidelines for Management of Hypertension, influenced by the results of the Systolic Blood Pressure Intervention Trial (SPRINT) recommends a reduction of the treatment target from 140/90 mmHg to 130/80 mmHg, including for the very old.17-19 However, some authors emphasised that there is a greater potential for harm to exceed benefit when BP targets are lowered.20
The European Guidelines for the Management of Arterial Hypertension recommend lowering systolic BP to <140 mmHg for all patient groups, including independent older patients, with a target of 130 mmHg for most patients if tolerated. That level is lower than the one recommended in previous guidelines. Even lower systolic BP levels (<130 mmHg) are recommended for some patients, especially to further reduce the risk of stroke. However, the European guidelines recommend against the reduction of systolic BP to <120 mmHg because of a possible increase in harm. According to the same guideline, BP targets in old and very old patients (above 80 years) with dependence, frailty and comorbidities may be higher.12
Blood Pressure Management for Patients with Stable Cardiovascular Disease
There is little evidence for the benefits in total mortality, serious adverse events, or total cardiovascular events for people with hypertension and cardiovascular disease treated to lower than target BP. Also, there is very limited evidence on adverse events associated with lower BP targets, which leads to high uncertainty. At present, evidence is insufficient to justify lower BP targets (≤135/85 mmHg) in people with hypertension and established cardiovascular disease. Further randomised clinical trials are needed to address this question.21
Hypertensive Urgencies and Emergencies
Hypertensive urgencies are situations associated with severe BP elevation in otherwise stable patients without acute or impending change in target organ damage or dysfunction.11 Many of these patients have withdrawn from or are noncompliant with antihypertensive therapy and do not have clinical or laboratory evidence of acute target organ damage. These patients should not be considered as having a hypertensive emergency and instead should be treated by reinstituting or intensifying their antihypertensive drug therapy and treatment of anxiety, as applicable.12,13 There is no indication for referral to emergency departments, immediate reduction of BP or hospitalisation for these patients.
Hypertensive emergencies are defined as severe elevations in BP (>180/120 mmHg) associated with evidence of new or worsening target organ damage. People with chronic hypertension can often tolerate higher BP levels than those who were previously normotensive. However, if the emergency is left untreated, the one-year death rate associated with hypertensive emergencies is higher than 79% and the median survival is 10.4 months.12,13
The most common emergency symptoms will depend on the organs affected but may include headache, visual disturbances, chest pain, dyspnoea, dizziness and other neurological deficits.22 Acute stroke, especially intracerebral haemorrhage, when associated with severe hypertension has often been termed a hypertensive emergency, but a more cautious approach is recommended for acute lowering of BP in the emergency setting for acute ischaemic stroke.12,14
Paradoxically, there is no evidence from randomised controlled trials that antihypertensive drugs reduce morbidity or mortality in patients with hypertensive emergencies. However, from clinical experience, it is highly likely that reduction of BP (not necessarily to normal) prevents or limits further target organ damage. There is also no robust evidence to suggest which first-line antihypertensive drug class provides more benefit than harm in hypertensive emergencies.12,13,22
For most hypertensive emergencies, IV administration of a short half-life drug under continuous haemodynamic monitoring is recommended to allow careful titration of the response to treatment. Esmolol, metoprolol, labetalol, fenoldopam, clevidipine, nicardipine, nitroglycerine, nitroprusside, enalaprilat, urapidil, clonidine and phentolamine are all recommended. In general, use of oral therapy is discouraged.11,13
If conditions requiring rapid lowering of systolic BP, such as aortic dissection or pheochromocytoma, are not present, the recommendation is to reduce blood pressure by a maximum of 25% over the first hour, then to 160/100–160/110 mmHg over the next 2–6 hours, then to normal over the next 24–48 hours.12
The survival of patients with hypertensive emergencies has improved dramatically over the past decades. However, these patients still have a high mortality risk and should be screened for secondary hypertension. Careful long-term follow up is also of utmost importance.11
Blood Pressure Management in Hypertensive Emergencies Involving Brain Damage
BP management in hypertensive emergencies involving brain damage (hypertensive encephalopathy, intracerebral hemorrhage and acute ischaemic stroke) should consider that the pathophysiology of brain damage is unique to each condition.12,14 Management should be tailored according to the disease and there is not a single recommendation that fits all. Consequently, the right diagnosis is crucial based upon clinical features, brain imaging, neurovascular evaluations and cardiac tests.
Hypertensive Encephalopathy
The diagnosis of hypertensive encephalopathy is based on the presence of vague neurologic symptoms, headache, confusion, visual disturbances, seizures, nausea and vomiting. The onset of symptoms usually occurs over 24–48 hours with neurological progression. The examination can show retinopathy (haemorrhages, exudates and papilledema), transient and migratory neurological nonfocal deficits ranging from nystagmus to weakness and an altered mental state ranging from confusion to coma.22,23 Complications can result in neurological deficits from intracranial haemorrhage. Focal neurological lesions are rare and should raise the suspicion of stroke.23
Symptoms are usually reversible with prompt initiation of therapy. It is usually safe to reduce mean arterial pressure by 20–25% and to lower the diastolic BP to 100–110 mmHg using labetalol, nicardipine or nitroprusside. Agents that affect the central nervous system, such as clonidine, reserpine and methyldopa, and diuretics should be avoided.13
Posterior reversible encephalopathy syndrome (PRES) has been increasingly recognised as a complication of hypertensive encephalopathy. Hypertension with failed autoregulation, dysfunction of the blood brain barrier, arteriolar dilatation and hyperperfusion leading to vasogenic oedema have all been implicated in its pathophysiology. The clinical presentation can be very similar to a hypertensive encephalopathy including headache, nausea, hemiparesis, hemianopsia, seizures and coma. Findings from brain MRI are typical and show symmetric hyperintensities in the subcortical white matter of the posterior temporal and occipital lobes in the fluid-attenuated inversion recovery sequences. Some patients can also present with string-of-beads and focal vasodilatation-vasoconstriction areas in the cerebral angiogram, a finding compatible with reversible cerebral vasoconstriction syndrome. However, PRES can also occur in patients without elevated BP levels, including those using immunosuppressive drugs, after organ and bone marrow transplantation and in patients with sepsis and multiorgan failure.24
Ischaemic Stroke
Acute ischaemic strokes occur due to an occlusion of an intracranial or cervical artery with consequent deprivation of blood and oxygen to a brain territory. A few minutes after an arterial occlusion in the brain, a core ischaemic lesion is established, however a larger area at risk of hypoperfusion can be salvageable if recanalisation therapies are administered. The salvageable area or ischaemic penumbra is largely dependent on collateral blood flow and acute reductions of BP can threaten perfusion in critical areas.25
In the acute phase of ischaemic stroke, early initiation or resumption of antihypertensive treatment is indicated only in patients treated with recombinant tissue-type plasminogen activator or if hypertension is extreme. For patients eligible for IV thrombolysis, antihypertensive treatment is recommended so that systolic blood pressure is ≤185 mmHg and diastolic blood pressure is ≤110 mmHg before treatment and <180/105 mmHg for the first 24 hours after treatment.14A recently published clinical trial evaluating more strict BP targets after IV thrombolysis for acute ischaemic stroke did not show long-term benefits in independence or survival, however lower BP levels were associated with lower rates of haemorrhagic transformation.26
The benefit of acute BP lowering in patients with acute ischaemic stroke who do not receive thrombolysis is uncertain. Initiation of treatment for these patients is suggested only if the systolic blood pressure is >220 mmHg or diastolic blood pressure is >120 mmHg or if the patient has another clear indication.14
Rapid reduction of BP, even to lower levels in the hypertensive range, can be detrimental. Therefore, if indicated, BP should be lowered cautiously, by about 15% during the first 24 hours after the onset of stroke.14
Patients with acute ischaemic stroke and a BP lower than 180/105 mmHg in the first 72 hours after stroke do not seem to benefit from the introduction or reintroduction of BP-lowering medication. For stable patients who remain hypertensive (≥140/90 mmHg) more than three days after an acute ischaemic stroke, initiation or reintroduction of BP-lowering medication should be considered. Restarting BP control is reasonable after the first 24 hours for hypertensive patients who are stable.14
Haemorrhagic Stroke
Spontaneous, non-traumatic intracerebral haemorrhage is the second most common cause of stroke after ischaemic stroke. The most common causes are hypertension, bleeding diatheses, amyloid angiopathy, drug misuse and vascular malformations.27
Subarachnoid haemorrhage is another subtype of haemorrhagic stroke. The two major causes of subarachnoid haemorrhage are rupture of arterial aneurysms that lie at the base of the brain and bleeding from vascular malformations that lie near the pial surface.28,29
In patients with intracerebral haemorrhage, BP is often elevated and hypertension is linked to greater haematoma expansion, neurological deterioration and worse prognosis. However, the management of hypertension is complicated by competing risks (reducing cerebral perfusion pressure in patients with intracranial hypotension) and potential benefits (reducing further bleeding).30,31
Intensive BP lowering (<140 mmHg) in patients with intracerebral haemorrhage had no clear benefits on clinical prognosis but was safe and associated with a modest better functional recovery in patients who survived a stroke in a large randomised clinical trial. A favourable trend was also seen toward a reduction in the conventional clinical end point of death and major disability.32 However, more intense BP lowering (<120 mmHg) not only did show not clinical benefits and was associated with more renal adverse events in another clinical trial using intravenous nicardipine.33 The American Heart Association guidelines recommend that for patients with intracerebral haemorrhage presenting with systolic BP 150–220 mmHg and without contraindication to acute BP treatment, acute lowering of systolic BP to 140 mmHg is safe and can be effective for improving functional outcome.27 A subsequent study, in which SBP was immediately reduced from a mean of 200 mmHg to two different target intervals (140–170 versus 110–139 mmHg), showed that more intensive BP lowering had no benefit on disability or death and was associated with more renal adverse events.34
Intracranial pressure is another important parameter to be considered in patients with intracerebral haemorrhage. If the systolic BP is higher than 180 mmHg and there is evidence or suspicion of elevated intracranial pressure, it is recommended to keep cerebral perfusion pressure at 61–80 mmHg. If there is no evidence or suspicion of elevated intracerebral pressure, a modest reduction of BP (160/90 mmHg) is recommended. If the systolic BP is 150–200 mmHg, acute lowering to 140 mmHg is probably safe.27 Drugs that may cause prolonged or precipitous decline in BP should be avoided.
The management of BP in the acute phase of subarachnoid haemorrhage is based on even less clinical evidence. Observational studies suggest that aggressive treatment of BP may reduce the risk of aneurysmal rebleeding, but with an increased risk of secondary ischaemia. Guidelines from different clinical societies agree that is reasonable to treat BP if the aneurysm is not yet secured, although the levels recommended in the guidelines differ. The American Stroke Association recommends <160 mmHg of SBP, the Neurocritical Care Society says <110 mmHg of mean arterial pressure,28,29 while the European Stroke Organisation found moderate-quality evidence to support weak recommendations for intensive lowering of SBP to <140 mmHg within 6 hours of intracraneal hemorragic stroke onset.35
Blood Pressure Management to Prevent Stroke Recurrence
About 25% of strokes are recurrent, the annual risk of recurrence is about 4% and the mortality rate after a recurrent stroke is 41%.12,13 In the North Dublin Population Stroke Study, the cumulative 2-year stroke recurrence rate was 10.8% and case fatality was 38.6%.36
The risk is also high after a transient ischaemic attack (TIA) or a minor ischaemic stroke. Data from a registry of TIA clinics in 21 countries that enrolled 4,789 patients showed that at 1-year follow-up, the rate of cardiovascular events including stroke was 6.4% in the first year and 6.4% in years 2–5.37
There are gaps in the evidence for the management of BP for secondary prevention of stroke and there is a need for further studies. BP-lowering therapy should be considered in patients with stable neurological status, 72 hours after onset of neurologic symptoms, or immediately after TIA, for previously treated or untreated patients with hypertension, except in patient with large vessel occlusion and fluctuating clinical symptoms.14,38
A Cochrane review of randomised controlled trials investigating BP-lowering treatment for the prevention of recurrent stroke, major vascular events and dementia in patients with a history of stroke or TIA. The BP-lowering drugs started at least 48 hours after stroke or TIA. The authors concluded that the results support the use of BP-lowering drugs in people with stroke or TIA for reducing the risk of recurrent stroke and that the current evidence is primarily derived from trials studying an ACE inhibitor or a diuretic and that no definite conclusions can be drawn from current evidence regarding an optimal systolic BP target after stroke or TIA.39
Reducing BP appears to be more important than the choice of agents and the effectiveness of the BP reduction diminishes as initial baseline BP declines. Angiotensin inhibitors, calcium channel blockers and diuretics are reasonable options for initial antihypertensive monotherapy and may be used in such patients. Beta-blockers should not be given unless there is a compelling indication for their use, particularly as the most common recurrent event after stroke is a further stroke rather than MI.39
The appropriate BP targets to prevent recurrent stroke are uncertain and depend upon the patient’s history.
- In patients with underlying hypertension, a goal BP of <140/90 mmHg or systolic pressure <130–135 mmHg is recommended by the current guidelines. A systolic BP level <130 mmHg was not associated with a lower stroke risk.38
- For patients with recent small vessel (lacunar) ischaemic stroke, lowering the systolic BP <130 mmHg may reduce the risk of a future intracerebral haemorrhage.40
- In patients with haemodynamically significant large artery disease, BP lowering should be used cautiously as tolerated, without a specific goal other than a minimum reduction of 10/5 mmHg.38
- In patients who develop recurrent neurologic symptoms referable to a stenotic artery when the BP is lowered below a threshold, the suggested management is to maintain BP above that threshold.38
Conclusion
Projections show that by 2030, an additional 3.4 million US adults aged ≥18 years, representing 3.88% of the adult population, will have had a stroke – a 20.5% increase in prevalence from 2012.41
BP is a powerful determinant of risk for ischaemic stroke and intracranial haemorrhage and there is evidence that controlling BP levels to <150/90 mmHg reduces the risk of stroke. Evidence of the benefits are weaker for lower BP targets obtained with intensive BP lowering, especially in older patients.42,43
The management of BP in adults with stroke is complex and challenging because of its heterogeneous causes and haemodynamic consequences. Future studies should focus on optimal timing and targets for BP reduction, as well as ideal antihypertensive agent therapeutic class by patient type and event type.
New strategies to identify and reduce stroke risk and improve management of acute stroke are necessary. Markers for increased risk may improve prevention. For example, in a study involving Chinese adults with hypertension, the subgroup with a low platelet count and high homocysteine levels had the highest risk of first stroke, and this risk was reduced by 73% with folic acid treatment.44
About 90% of the stroke burden is attributable to modifiable risk factors, with about 75% being due to behavioural factors such as smoking, poor diet and low physical activity. Achieving control of behavioural and metabolic risk factors could avert more than three-quarters of the global stroke burden.45
Health promotion strategies for positive cardiovascular health should be emphasised, in addition to the treatment of established CVD. The concept of cardiovascular health is characterised by seven metrics (Life’s Simple 7 defined by the American Heart Association).46 Ideal cardiovascular health involves the absence of clinically manifest CVD together with the simultaneous presence of optimal levels of all seven metrics, including not smoking and having a healthy diet pattern, enough physical activity, a healthy body weight and normal levels of total cholesterol, blood pressure and fasting blood glucose, in the absence of drug treatment.46
Unfortunately, the number of people – even young people – who have far from ideal cardiovascular health is still high. A survey of 550 physicians in 11 European countries found that primary care doctors reported that less than 30% of patients >40 years old were screened for blood pressure, whereas even fewer were screened for AF.47
Programmes to improve patients’ and health providers’ education and communication are needed, and several are being studied and show promise.48 Patients should increase adherence to medical treatments and adopt a healthy lifestyle. Healthcare providers should have tools for quality improvement interventions on adherence to evidence-based therapies.49
Primordial prevention strategies that prevent the emergence of stroke risk factors should be the ultimate goal. Measures such as salt reduction and dietary interventions, implementation of tobacco control and support to the development of healthy environment are crucial for reducing the burden of cardiovascular diseases. This endeavour needs close collaboration between healthcare professionals, institutions and governments.