Aortic stenosis (AS) is the most common valvular heart disease in both women and men in developed countries, with a median age at diagnosis peaking in the 8th decade of life, and approximately 6 years later in women than in men.1 With longer life expectancy and decreasing complications related to other cardiovascular diseases and in particular coronary artery disease, the AS burden on the health system is increasing.2 This in turn puts the focus on correct grading and patient selection for newer treatment modalities, including endovascular therapies, which have become increasingly available during the past decade, and that improve the survival and quality of life of older patients.
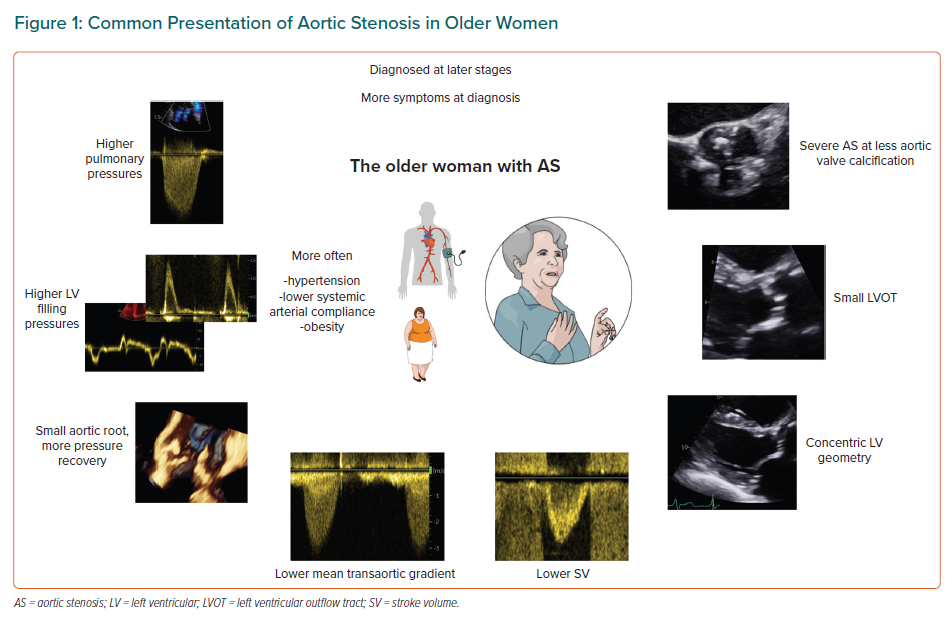
Women and men have different AS severity grades at the same level of valve calcification, and specific patterns of left ventricular (LV) structural and functional remodelling in response to the chronic pressure overload.3–5 Despite similar rates of AS progression, women are less often referred to interventions than men and at a later stage of disease, which contributes to the observed excess mortality after surgical aortic valve replacement.5–7 These striking sex gaps are not accounted for in the new European guidelines that do, however, acknowledge that we lack evidence on indications and timing of treatment in women versus men with valvular heart disease.8 Here, we review the challenges in grading AS in women that might contribute to these differences in management and outcome (Figure 1).
Structural Changes of the Aortic Valve
Although the pathogenesis of AS is complex, and involves mechanical injuries, active inflammation and osteoblastic differentiation, with consequent progressive fibrosis and calcification, it is the calcium deposition that has been regarded as the hallmark of AS, and indeed recognised as strongly predictive of rapid AS progression and death.3,9 Valve calcification is a simple, flow-independent measure of AS severity, and can be assessed semiquantitatively at the routine transthoracic echocardiography or quantified by the Agatston score using non-contrast CT.3,10,11 By the latter, the significantly lower amount of calcium in female compared with male aortic valves, at the same haemodynamic severity of AS, has been recognised, and two different thresholds of valve calcification identifying severe AS as likely in women (1,200 AU) and men (2,000 AU) are now recommended.3,12,13 Through a continuum of risk, the higher the Agatston score, the higher is the probability of severe AS, but with much lower calcium valve loads necessary in women to achieve a severe valve obstruction.
The method is highly reproducible, quantitative and recommended by guidelines in cases of discordant AS grading, in particular in patients with low-flow, low-gradient AS with aortic valve area (AVA) ≤1 cm2, but ejection fraction (EF) ≥50%. However, it is not a perfect measure of the severity of AS, as it does not provide information on valve morphology, the calcium distribution in the valve or the amount of leaflet fibrosis. Based on small-scale studies, the latter has been suggested to contribute more to leaflet restriction in women than in men. Aortic valves in women demonstrate higher content of dense connective tissue than aortic valves in men at the same degree of AS severity.14 Assessment of non-calcific leaflet thickening by contrast-enhanced CT has been proposed as a surrogate measure of valve fibrosis, and combined fibrocalcific changes using this method have been shown to correlate better with peak aortic velocity than the calcium score alone in a small group of women with AS.15 However, this approach needs validation in larger cohorts, and threshold values for the amount of fibrosis identifying severe AS need to be defined in both sexes. PET/CT with a tracer specific for activated fibroblasts and, thus, developing fibrosis might improve fibrosis detection in the aortic valves, and allow its quantification in female versus male valves.16 However, this technique is still under development, and the use of PET/CT in clinical practice remains challenging due to, among others, high costs and limited access. In the meantime, it is worth keeping in mind that in the average woman with non-discordant AS where CT calcium score is not routinely performed, a simple visual assessment of how calcified the valve is would in most cases underestimate the degree of AS and contribute to later referral to intervention.
Left Ventricular Outflow Tract and Stroke Volume
Echocardiographic measurement of mean transaortic pressure gradient, peak transaortic velocity and calculation of effective AVA based on the continuity equation are still the core of diagnosis and assessment of AS severity. The continuity equation is based on the simple concept that the forward stroke volume (SV; the SV ejected in the LV outflow tract; LVOT) must be equal to the SV at the level of the aortic valve orifice:
SVLVOT = SVaortic valve
As the volume of a circular space is equal to the cross-sectional area multiplied by the velocities during the ejection period (i.e. the velocity time integral), stroke volumes on both sides of the equation can be written as the product of area and velocity time integral. Thus, both the forward SV (and implicitly the cardiac output) and the severity of AS can be obtained by tracing the Doppler signals registered in the LVOT and through the aortic valve, and by measuring the LVOT size. As simple as this sounds theoretically, this approach has several practical drawbacks, among others that assessment of both SV and AVA rely heavily on the correct measurement of the LVOT diameter.
The normal LVOT has an ovoid shape, with the transverse diameter larger than the antero-posterior diameter throughout the cardiac cycle.17 At echocardiography, the LVOT diameter is recommended to be measured on a parasternal long-axis view at the annular level ‘inner edge to inner edge’; that is, between the hinge points of the aortic cusps.18 No normal reference values have been defined for the LVOT size, but it has been established that the LVOT diameter increases linearly with the body surface area by a 5.7 coefficient.19 Thus, women, by their smaller body size, have smaller LVOTs than men. However, in healthy populations, the LVOT area remains smaller in women than in men even after adjustment for body surface area.17 Women have also on average more often small AVAs than men, even in the absence of valvular pathology.20 Having smaller LVOTs and valves can represent an intraoperative challenge, increasing the complexity of surgical valve replacement, but also has consequences for AS grading. Putting the sex differences in size in the continuity equation translates, namely, into women having either lower SVs than men at the same peak velocity and mean gradient value or increased velocities across the valve/increased mean gradient at the same SV value as men.
In healthy individuals, women have indeed lower SVs than men, even after adjustment for body size.21 This is physiologically compensated by a higher heart rate to maintain a normal cardiac output. Calculation of SV as a necessary step in the assessment of AS severity is obviously important, as the degree of aortic valve opening is directly dependent on the ejected blood volume, and low SV has been extensively documented as a negative prognostic factor in AS.22,23 However, using the same cut-off value of 35 ml/m2 for defining low SV as a surrogate of low flow in both women and men, as current guidelines recommend, seems biologically counterintuitive and probably contributes to the described higher prevalence of low-flow AS with preserved EF in women.8,24 So far, only one study in AS has used sex-specific thresholds for SV and demonstrated that a 32 ml/m2 cut-off in women and 40 ml/m2 in men improves prediction of mortality after aortic valve replacement.25 A recent review on sex differences in valvular heart disease also promotes the use of sex-specific cut-offs for SV indexed for body size in the assessment of patients with low-flow, low-gradient AS.26
The Aortic Root and Pressure Recovery
The LVOT size is positively correlated with the aortic root size, and, likewise LVOT, women more often have smaller aortic roots than men.27,28 A smaller aortic sinotubular junction increases the pressure recovery in AS causing enhanced transformation of some of the kinetic energy to potential energy upstream the aortic valve.27 This in turn results in a lower pressure drop through the valve, meaning that at the same AVA, a woman with a small aortic root will have a lower mean gradient than a man with a larger ascending aorta. Moreover, a small root <1.4 cm/m in women is a marker of higher ischaemic risk and higher cardiovascular mortality in asymptomatic AS.28 Adjustment of AVA for pressure recovery, after indexation for body surface area, significantly improved the prediction of aortic valve-related events, as well as of combined total mortality and hospitalisations for heart failure, in 509 AS patients with small aortic roots included in the SEAS trial.29 Therefore, as recommended by the European Association of Cardiovascular Imaging and the American Society of Echocardiography, pressure recovery should be taken into account primarily in patients with a diameter of the ascending aorta <30 mm.18
In women with narrow aortic roots, one should therefore consider additional grading criteria besides the standard haemodynamic assessment, among others the CT valvular calcium score.
Interestingly, in a cohort of patients with advanced aortic valve disease and/or ascending aortic aneurysm, but devoid of coronary artery disease, referred for surgery at the Karolinska University Hospital between 2007 and 2017, women had smaller absolute ascending aortic dimensions, but larger aortic dimensions normalised for body surface area.30 This can explain the observed higher aneurysm growth rate in women with implicit higher risk of complications and rupture, and underlines the importance of normalising aortic dimensions to body size.31 In the same cohort, among patients with bicuspid aortic valves, women more often had AS and less aortic regurgitation compared with men, suggesting sex-specific molecular mechanisms in the development of bicuspid aortic valve disease.30
The Left Ventricular Adaptation and Ventriculo–arterial Coupling
The LV remodelling during AS progression differs in women and men, with men developing larger LV masses and more LV hypertrophy, even when hypertrophy is defined using sex-specific cut-offs.4,32 Women, in contrast, present smaller ventricles with more often concentric geometry both at milder disease stages and in severe AS.4,33,34
The direct consequences of this type of remodelling are smaller LV volumes and SVs based on volumetric calculations, numerically higher endocardial fractional shortening and LV EF due to enhanced endocardial displacement, and increased filling pressures.35
EF remains normal up to very advanced AS stages and higher in women, despite a progressive reduction in midwall systolic function and global longitudinal strain, with negative prognostic implications.5,36,37 Of note, healthy women have higher EF than men (lower limit of normal 54 versus 52%), meaning that using the classic guideline indication for intervention of LV EF <50% in severe, asymptomatic AS will select women with more advanced disease than men.38 However, the 2021 guidelines open for the possibility to reffer to intervention also asymptomatic patients with severe AS and EF <55% under a class IIa level B indication.
Smaller LV cavities with higher LV filling pressures have been demonstrated in women with comparable AS severity with men and are linked to earlier symptom onset.39 This might reflect both the sex-specific LV remodelling, as well as the higher prevalence of comorbidities associated with heart failure with preserved EF in women, in particular obesity and hypertension.7,40 Interestingly, in patients with low-gradient AS, among which women are overrepresented, a sharp increase in pulmonary capillary wedge pressure with exercise was the main determinant of functional status, and it was not related to resting AVA, mean gradient or SV.41 Approximately 70% of the patients included in the study, with an average baseline AVA of 0.71 cm2, achieved an AVA >1 cm2 at peak exercise, suggesting that low-gradient AS is a heterogenous patient group with multifactorial symptom causes.41
The proportion of hypertrophy–fibrosis in the enlarged LV mass due to chronic pressure overload in AS has been explored in several experimental and clinical studies, generally of smaller scale, with the largest body of evidence indicating increased focal fibrosis (detected at cardiac magnetic resonance by late gadolinium enhancement) and diffuse fibrosis (detected by T1 mapping at cardiac magnetic resonance or in myocardial biopsies) in men.33,34,39,42 Thus, a maladaptive LV response with more LV hypertrophy, increased profibrotic gene expression and increased fibrosis are more common in men during progression of AS. However, the presence of a preoperative maladaptive LV remodelling has been found to be associated with reduced survival in women, but not in men, 4 years after aortic valve replacement, suggesting a stronger negative impact in women than in men.33 Cardiac magnetic resonance is not routinely performed in AS patients, and the only aspect of LV structural remodelling included in the European valve guidelines is severe LV hypertrophy as a possible indicator of need for earlier intervention in asymptomatic patients. The use of this prognostic marker will naturally select several more men than women for intervention, and in this context, it is worth remembering that small, non-hypertrophic LVs do not exclude advanced AS in women.
Finally, women with AS are older, and have a higher prevalence of hypertension and more often low systemic arterial compliance than men.43 Both higher blood pressure and stiffer arteries contribute to higher valvulo-arterial impedance, lower measured transaortic pressure gradients and increased mortality.43 The guideline recommendation is to re-evaluate the patient with AS when a normotensive state is achieved, but this is challenging to apply in clinical practice.8
The Right Ventricular Adaptation
In healthy individuals, it has been demonstrated that women have better right ventricular (RV) systolic function than men, also after adjustment for body size.44 Sex differences in the RV adaptation to AS have been little addressed. In contrast to the LV, RV remodelling is regarded as a late manifestation of AS. Based on data from the PARTNER 2 trial, a staging of the total cardiac damage in severe AS was proposed in 2017, with RV damage classified as the last stage (stage 4) of damage, and changes in the pulmonary vasculature with estimated systolic pulmonary pressure ≥60 mmHg as stage 3.45 Men were overrepresented in the stage 4 group, but male sex did not independently predict 1-year outcome in that study. In a larger cohort of patients referred to transcatheter aortic valve implantation, more women than men had stage 3 and 4 cardiac damage, but the relationship of different stages to outcome was not assessed in sex-specific analyses.46 Large AS studies showed, however, that women are referred later to surgery than men and, at similar haemodynamic AS severity, they have higher pulmonary pressures, are more symptomatic and incur higher mortality.6
Grading of Aortic Stenosis and Sex Disparities in Aortic Stenosis Management
Analyses of contemporary, prospectively collected data in large populations of AS patients show persistent sex disparities in referral to valve intervention. Women are older when they achieve a similar haemodynamic AS severity as men based on routine assessment of peak aortic jet velocity, mean gradient and AVA, and less likely to be referred to valve intervention.7 Women with discordant low-gradient AS register excess mortality, stressing the diagnostic challenges this AS subtype poses when using the presently recommended guidelines criteria.7 Interestingly, in transcatheter aortic valve implantation studies, the mortality excess in women persisted in those with pulmonary hypertension at the time of diagnosis, but was blunted in those with normal pulmonary pressures, possibly due to the more active use of CT valve calcium score in the diagnostic work-up of patients referred to transcatheter aortic valve implantation compared with surgical aortic valve replacement.47 Implementation of sex-specific thresholds in the haemodynamic assessment of AS, including of SV normalised for body size, in combination with sex-specific aortic valve calcium thresholds in the routine work-up of patients with more than mild AS will potentially contribute to earlier identification of women with low-gradient severe AS that have an indication for intervention. In patients that are overweight or obese, one might consider normalisation of SV and AVA for height at the correct allometric power rather than body surface area, even if the prognostic effect of this approach in AS is yet to be demonstrated.48 Moreover, in patients with small aortic roots for body size, one should be suspicious of the contribution of pressure recovery to the lower transvalvular gradients and verify that by the additional use of the energy loss index.29
Conclusion
Sex differences in valvular, ventricular and arterial factors have been documented, and make assessment of AS more challenging in women. We encourage the use of sex-specific thresholds in the haemodynamic assessment of AS patients, and of correct normalisation for body size to reduce these challenges in clinical practice. A multimodality approach including CT and PET/CT with specific fibrosis and microcalcification tracers might in the future further improve grading of AS in women and men, and push forward development of sex-specific recommendations in AS.