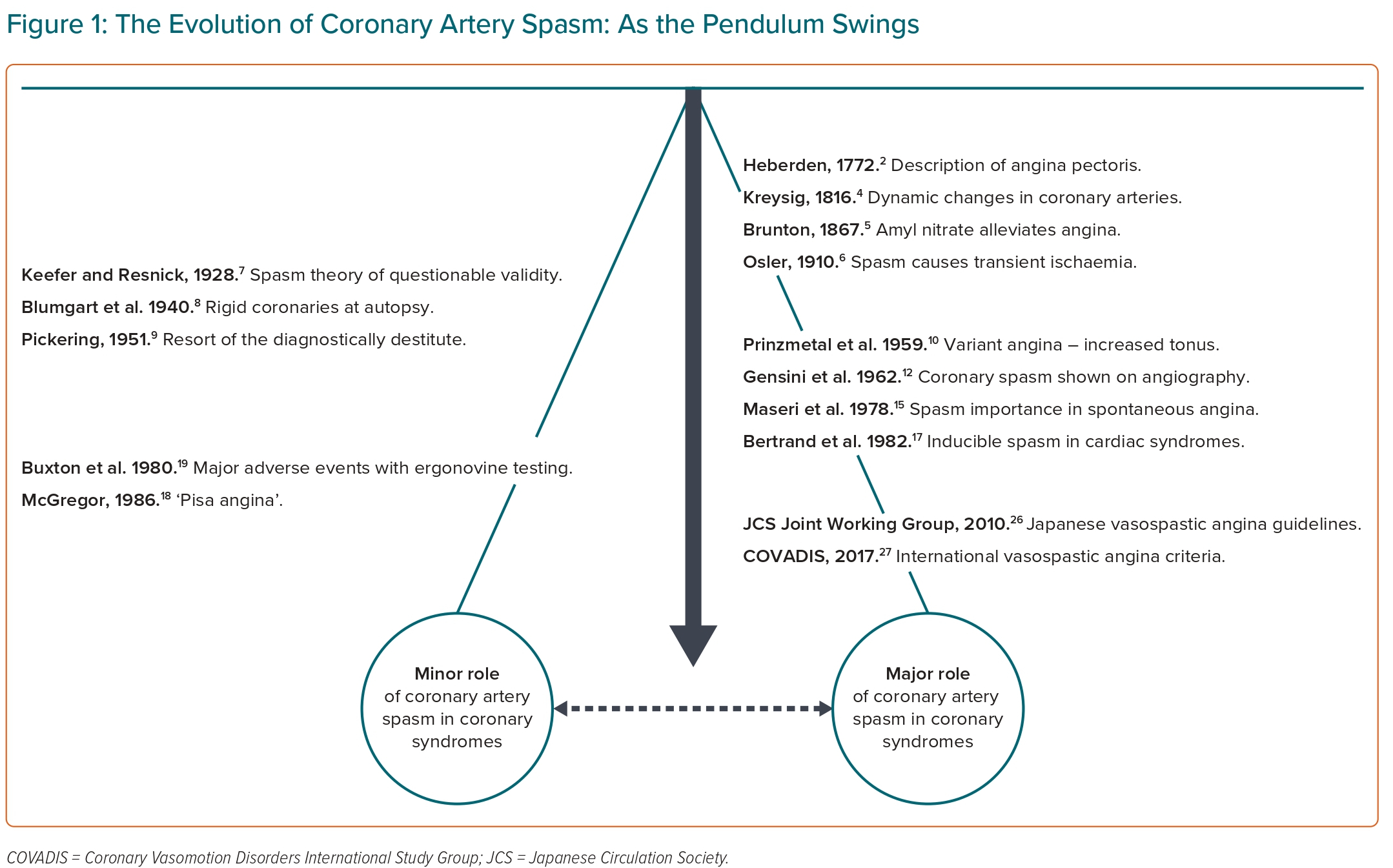
The concept of coronary artery spasm was described more than 200 years ago; however, during this time the concept has had a troublesome course, with the pendulum of scientific opinion swinging from ‘unbelievers’, who rationalised it did not exist or played only a minor role in coronary syndromes, to the avid ‘believers’, who undertook clinical studies supporting its major importance in coronary syndromes (Figure 1). The challenge in identifying the role of coronary artery spasm in coronary syndromes relates to its transient nature and difficulty in imaging its presence during spontaneous episodes. As an iconic clinician scientist, Prof Attilio Maseri demonstrated the importance of coronary artery spasm in the modern era and is considered by many to be the guru of this intriguing pathophysiological condition. In this special edition of the journal, the ‘pendulum-swinging’ evolution of the coronary artery spasm will be reviewed, with special reference to Maseri’s contribution. The importance of understanding the historical perspective of coronary artery spasm is reflected in the words of the American–Spanish philosopher George Santayana: “Those who cannot remember the past are condemned to repeat it.”1
Origins of the Concept of Coronary Spasm
Thanks to knowledge gained over the centuries, any lay member of the public will tell you that chest pain may arise from the heart. In 1772, William Heberden provided an eloquent description of what he called ‘angina pectoris’, which is still accurate today and the key symptom for myocardial ischaemia regardless of the underlying cause.2 In his seminal paper Some Account of a Disorder of the Breast,2 he writes:
“But there is a disorder of the breast marked with strong and peculiar symptoms, considerable for the kind of danger belonging to it, and not extremely rare, which deserves to be mentioned more at length. The seat of it, and sense of strangling, and anxiety with which it is attended, may make it not improperly be called angina pectoris. They who are afflicted with it, are seized while they are walking, (more especially if it be up hill, and soon after eating) with a painful and most disagreeable sensation in the breast, which seems as if it would extinguish life, if it were to increase or continue; but the moment they stand still, all this uneasiness vanishes.”
This description is now considered as classical exertional angina, although Heberden did recognise that angina pectoris may also occur at rest, as reflected in his comment “Such is the most usual appearance of this disease; but some varieties may be met with. Some have been seized while they were standing still or sitting; also, upon first waking out of sleep.” Thus, his comprehensive description unknowingly detailed some of the features of rest angina associated with coronary artery spasm. However, despite his detailed descriptions reflecting his astute clinical acumen, Heberden did not attribute ‘angina pectoris’ to a disorder of the heart, commenting that his autopsy studies found “no fault in the heart, in the valves, in the arteries, or neighbouring veins, excepting some small rudiments of ossification in the aorta.” The clinicopathological link between angina pectoris and obstructive atherosclerotic coronary artery disease is attributed to autopsy studies undertaken by Caleb Parry in 1799,3 who ascribed the findings to his colleague, Edward Jenner (the pioneer of the smallpox vaccine).
Although providing a structural pathophysiological mechanism for angina pectoris, with tight atherosclerotic coronary artery lesions obstructing coronary blood flow during exercise resulting in myocardial ischaemia and thus symptoms, some autopsies studies of angina patients did not have significant coronary artery disease. This prompted consideration of functional pathophysiological mechanisms, with Friedrich Kreysig in 1816 speculating upon dynamic changes in the coronary arteries; a speculative conclusion considering that 19th century technology was unable to confirm such a theory.4 Also noteworthy, Brunton in 1867 reported that angina pectoris could be alleviated with amyl nitrate, supporting a dynamic element to this disorder.5 However, it was not until the 1910 Lumleian Lectures by Sir William Osler that the concept of coronary artery spasm was firmly established in medical practice.6 Thus, by the early 20th century, the pendulum had swung strongly in favour of coronary artery spasm being a key mechanism in coronary syndromes.
The Demand Ischaemia Concept
By the mid 20th century, the pendulum began to swing in the other direction, with influential clinicians such as Chester Keefer and William Resnik reporting in 1928 that the rigid atherosclerotic lesions surely could not physically develop spasm.7 This was further supported in 1940 by Herrman Blumgart’s clinicopathological studies, where he reported that atherosclerotic coronary artery disease was responsible for angina in all his autopsy patients.8 Moreover, he demonstrated the importance of coronary collaterals in patients with occluded vessels without symptoms of angina, as well as coronary thrombosis in those with acute MI. Finally, the coronary artery spasm pendulum swung to an extreme antagonistic view in 1951, when George Pickering referred to a diagnosis of coronary artery spasm as “the resort of the diagnostically destitute”.9
The concept that angina pectoris could only arise from obstructive atherosclerotic coronary artery disease promulgated the ‘demand ischaemia’ concept, where myocardial oxygen demand during exertion exceeded the limited blood supply, thus resulting in myocardial ischaemia and, therefore, angina. Thus, angina pectoris could only arise from an increased myocardial oxygen demand due to an increased heart rate, blood pressure or myocardial contractility. Acute MI arises from excessive demand or a further reduction in blood supply from progression of atherosclerosis or coronary thrombosis. As clinicians at the time did not have the tools to treat coronary atherothrombotic conditions, therapy focused on reducing this demand ischaemia. Thus, acute MI patients were subjugated to 6 weeks strict bed rest in order to minimise myocardial oxygen demand; a strategy that possibly produced more deaths via immobilisation-associated deep venous thrombus and acute pulmonary embolism than it saved.
Prinzmetal’s Variant Angina
In 1959, Myron Prinzmetal described 32 cases that did not conform with the demand ischaemia concept as the patients had a preserved exercise tolerance and the angina symptoms occurred at rest rather than on exertion.10 Furthermore, whereas effort angina was associated with transient ST depression, this new form of rest angina was associated with transient ST elevation. He referred to this new syndrome as variant angina, with the key characteristics including:
- Angina at rest, which was often more severe and prolonged than effort angina but was nitrate-responsive, recurrent, with a waxing and waning cyclical pattern, frequently occurred at the same time of day and patients typically had a preserved exercise tolerance.
- Transient ST elevation, usually in the territory of a large coronary artery and may be the same site of future myocardial infarct.
- Frequent ventricular arrhythmias during the acute episode.
Prinzmetal ascribed the variant angina episodes to a ‘temporary increased tonus of a large narrowed coronary artery’, avoiding the term ‘spasm’ because it was a loathed term at that time. Of note, Prinzmetal reported the presence of atherosclerotic coronary artery disease in most autopsies of his variant angina patients, so that the ‘spasm’ occurred in the context of atherosclerosis. Indeed, it was not until 1973, when variant angina was reported as occurring in the absence of atherosclerotic coronary artery disease; the so-called ‘variant of the variant’.11
The remarkable accomplishment of Prinzmetal and his preceding advocates for coronary artery spasm was that their hypotheses were primarily based upon clinical deduction as coronary spasm could not be visualised but only inferred from the limited clinical tools and autopsy findings available in that era. This changed in 1962 with the advent of invasive selective coronary angiography when Goffredo Gensini angiographically demonstrated transient coronary artery spasm (in a patient with moderate coronary atherosclerosis) during an episode of rest angina, with both the pain and spasm alleviated by nitrate administration.12 With the ability to image coronary spasm during angiography, the field could make further advances and thus the pendulum swung further towards the importance of coronary artery spasm.
Vasospastic Myocardial Ischaemia: A Continuous Spectrum
As an astute clinician scientist, Maseri used multiple diagnostic investigations to understand the pathophysiological events occurring during spontaneous episodes of rest angina in patients admitted to the coronary care unit in Pisa, Italy. In particular, they undertook continuous ischaemic ECG monitoring (ST/T wave changes) in all patients and continuous haemodynamic measures for myocardial oxygen demand (i.e. heart rate, aortic blood pressure, right/left ventricular pressures and myocardial contractility) in patients with frequent episodes of angina. Furthermore, myocardial scintigraphy and coronary angiography were undertaken during spontaneous episodes of rest angina, thereby evaluating the impact on myocardial perfusion and the presence of coronary artery spasm.
His initial focus was on patients with variant angina (rest angina with transient ST elevation) and confirmed what Prinzmetal had speculated: the spontaneous episodes of rest angina associated with ST elevation were not preceded by an increased myocardial oxygen demand (i.e. increased heart rate, blood pressure or contractility) but a reduced myocardial perfusion in the coronary territory that subtended the vessel with angiographic confirmed coronary artery spasm.13,14 Moreover, the novel continuous ischaemic ECG monitoring demonstrated that these patients also had some episodes with ST depression and on occasions the ischaemic ECG changes may occur in the absence of symptoms (i.e. silent ischaemia).
This preliminary observation of variant angina patients also having rest angina episodes with ST depression suggested that coronary artery spasm was more ubiquitous than first thought and inspired Maseri to investigate the role of coronary artery spasm beyond variant angina. In 1978 he published his group’s experience of 138 comprehensively assessed patients admitted with rest angina (now referred to as unstable angina) and established that variant angina was only “one aspect of a continuous spectrum of vasospastic angina”.15 The fundamental principles from this iconic study influenced clinical practice for many years and demonstrated:
- The utility of continuous ischaemic ECG monitoring, with recordings pre-/post-nitrate administration in patients with recurrent rest angina increasing the recognition of variant angina.
- The direction of ST change (elevation or depression) does not correspond to the pathogenetic mechanism of ischaemia (i.e. spasm-induced impaired supply versus demand ischaemia) but to differences in the extent and distribution of ischaemia.
- That the term ‘vasospastic angina’ encompassed a broader concept (i.e. ST elevation or depression) of coronary artery spasm-induced ischaemia.
- The rest angina is not preceded by an increased myocardial oxygen demand, as evident on continuous haemodynamic monitoring.
- Novel insights into the ischaemic cascade, where haemodynamic ventricular relaxation and contraction abnormalities occurred before the onset of ischaemic ECG changes and chest pain was consistently the last occurrence in the cascade.15
Maseri subsequently extended the vasospastic angina spectrum to acute MI, demonstrating that the same coronary artery spasm territory could later evolve into infarction.16
To further evaluate the spectrum of coronary artery spasm in different cardiac disorders, Michel Bertrand performed provocative spasm testing in 1,089 consecutive patients undergoing coronary angiography for clinical indications varying from atypical chest pain to recent acute coronary syndrome.17 They reported on the prevalence of methylergometrine-induced coronary artery spasm during angiography, observing a low prevalence in patients with non-coronary disorders (i.e. 1.2% in those with atypical chest pain, 2% in valvular heart disease and 0% in cardiomyopathy), a moderate prevalence in stable coronary disorders (i.e. 4.3% in stable exertional angina and 6.2% in those with an old myocardial infarct occurring more than 6 weeks prior to angiography) and a high prevalence in those with more acute coronary disorders (i.e. 38% of patients with rest angina, 20% of those with an acute myocardial infarct within the past 6 weeks and 13.8% of those with both angina on effort and at rest). Unlike the studies undertaken by Maseri, these were not performed during spontaneous episodes of chest pain, but supported the concept of a continuous spectrum of vasospastic myocardial ischaemia.
The concept proposed by Maseri for a spectrum of pathophysiological mechanisms was a paradigm shift in the management of ischaemic heart disease. Diagnosis and treatment should not only consider obstructive atherosclerotic coronary artery disease and strategies to reduce myocardial oxygen demand (i.e. bed rest), but also the role of coronary artery spasm and coronary thrombosis and, therefore, strategies that improve myocardial oxygen supply (i.e. anti-spasm agents and anti-thrombotic agents).
The Era of Revascularisation and Reperfusion
The interest in coronary artery spasm garnered by Maseri started to diminish in the US by the mid-1980s, when the pendulum began to swing towards coronary spasm having a minor role in coronary syndromes. Firstly, US clinicians did not observe many cases of variant or vasospastic angina and referred to the phenomenon as ‘Pisa angina’.18 Secondly, bedside ergonovine provocative spasm testing was reported to produce major adverse cardiac events, including death, thereby limiting its use in the diagnosis of coronary artery spasm.19 Thirdly – and possibly most importantly – the role of coronary thrombosis in acute MI became evident and the effectiveness of thrombolytic agents in restoring reperfusion was established.
Furthermore, percutaneous revascularisation therapies emerged, which have been very effective in the management of acute coronary syndromes in conjunction with anti-thrombotic therapies. The effectiveness of these strategies shifted the focus towards atherothrombotic processes, with vasospastic mechanisms being overshadowed. However, these revascularisation strategies have less of an impact on chronic coronary syndromes, with as many as 40% of these patients continuing to experience angina despite technically successful percutaneous revascularisation.20
Despite the declining interest in coronary artery spasm in the US during the 1980s and 1990s, interest was maintained in specialised European centres.21 Maseri continued to research this challenging disorder, demonstrating the benefits of calcium channel blockers such as verapamil and exploring novel pharmacological provocative spasm agents as a method of understanding the pathophysiological mechanisms responsible for coronary artery spasm.22 He also expanded his interest into coronary microvascular dysfunction, recognising that this was also an under-appreciated mechanism of angina. Many of these concepts are captured in his 1995 textbook Ischaemic Heart Disease.23
In contrast to the declining interest in coronary artery spasm within the US, it was fervent in Japan, with studies evaluating associated clinical characteristics and the development of a coronary angiogram-based provocative spasm testing protocol using intracoronary acetylcholine, which has been routinely used in many Japanese centres for patients with suspected angina. These prolific research studies suggested that coronary artery spasm may be more prevalent among Japanese people. This prompted Maseri to initiate a clinical trial comparing Japanese and Italian MI patients, demonstrating inducible spasm 2 weeks following acute MI in 80% of Japanese and 37% of Italian patients.24 Thus, by the end of the 20th century, interest in coronary artery spasm was maintained in specialised European centres and bountiful in Japan.
Contemporary Guidelines
The experience and enthusiasm among Japanese clinicians for the diagnosis and management of coronary artery spasm have seen many innovative studies in this field emanate from Japan, including the molecular mechanisms for coronary artery spasm, predisposing risk factors and prognostic factors.25 Moreover, in 2010, the Japanese Circulation Society Joint Working Group established the first clinical guidelines for the diagnosis and treatment of vasospastic angina, which, for the first time, brought together the collective contemporary knowledge in this field.26
At an international level, the Coronary Vasomotor Disorders International Study (COVADIS) group subsequently developed diagnostic criteria for vasospastic angina, providing the nomenclature to enable international studies to be undertaken of these heterogeneous disorders.27 Of note, Maseri attended the first two annual COVADIS summits (2013 and 2014) and provided advice in the development of international guidelines on coronary spasm.
Another important recent clinical advance in coronary artery spasm was the CorMICA study.28 This trial demonstrated that functional coronary angiography to assess the presence of coronary artery spasm and/or coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease, had better outcomes with an appropriate stratified treatment plan than control patients who received standard care. These findings have prompted the inclusion of provocative spasm testing in both European and American guideline recommendations.29,30
Hence, in contemporary medicine, the assessment of coronary artery spasm has been incorporated in clinical guidelines, so that the pendulum is once more swinging in favour of coronary artery spasm. Moreover, in recent years, interest has evolved in ‘microvascular spasm’, where provocative coronary spasm testing does not invoke large vessel spasm but produces chest pain and ischaemic ECG changes, thereby implicating spasm at the microvascular level.31 The characteristics and management of these patients are not as well described as for those with large vessel spasm; future studies will clarify these aspects of this intriguing phenomenon.
Future Considerations in the Evolution of Coronary Artery Spasm
Unfortunately, in 2021, the world lost an outstanding mentor and clinical scientist with the passing of Attilio Maseri and tributes to his scientific contributions were echoed from international icons across the world.32 If he were still with us today, what advice would he give in relation to the further evolution of coronary artery spasm? As students of his mentorship, the authors would speculate he might continue to advise us to:
- “Consider every patient you see as an n=1 research study, trying to dissect the clinical presentation and understanding the underlying pathophysiological mechanism.”
- “Look for coronary artery spasm, since if you don’t, you will miss the diagnosis;” hence, promote the appropriate use of guideline-recommended provocative spasm testing.
- “Always continue reading the scientific literature and seek new technologies to facilitate clinical research into this puzzling disorder.”
Certainly, medicine and patients have benefited from Maseri’s contribution to the diagnosis and management of coronary artery spasm. It is up to future generations to heed the lessons learned and avoid George Santayana’s prediction that “those who cannot remember the past are condemned to repeat it.”