Heart failure (HF) is a clinical syndrome that arises from a variety of heart diseases.1 The American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) define HF as a complex clinical syndrome resulting from structural or functional disturbances of ventricular filling or blood ejection.2 There are some functional symptoms that appear in patients with HF, such as decreased aerobic capacity, decreased muscle strength, a dramatic decrease in physical activity and intolerance to moderate physical activity, which are accompanied by symptoms of fatigue and dyspnoea.3,4 The majority of patients with HF with preserved ejection fraction (HFpEF) have reduced ability to perform daily activities even though they have good contractility of the myocardium and good systolic function parameters, and they have a reduced quality of life.5,6 Clinical studies have also shown that maximal aerobic capacity is inversely correlated with the severity of HF and is directly correlated with the patient’s prognosis and life expectancy.7,8
The physical limitations and psychological stress on patients with HFpEF are considerable and they have a 5-year mortality (from diagnosis) of approximately 50%.9 Various evidence-based treatment options, such as neurohumoral drugs, device therapy and cardiac rehabilitation, have improved survival rates in systolic heart failure (SHF) or HfrEF patients, but no single treatment has consistently improved the prognosis in HFpEF in the general population.10
Ranolazine is a well-tolerated drug that selectively blocks sodium channels (INa). In addition, ranolazine has beneficial metabolic properties and does not affect heart rate or blood pressure. Ranolazine is currently approved in the US and Europe as a second-line agent in the management of chronic coronary syndrome.11 Oral ranolazine has peak plasma concentration within 1 hour after administration and has a bioavailability range of 35–45%.12 About 63% ranolazine is bound to protein plasma and its volume of distribution ranges from 85–180 l at steady state.12 Most of the drug is extensively metabolised by cytochrome P450 (CYP) 3A enzyme and primarily excreted in urine (75%).13
It may increase simvastatin and digoxin concentration by inhibiting CYP3A and P-glycoprotein, respectively.12 In the steady state, the half-life time of ranolazine is about 7 hours.14 The pharmacokinetics of ranolazine is not influenced by age, sex, diabetes or congestive HF.15 Although congestive HF is associated with changes in hepatic blood flow, as the pharmacokinetics of ranolazine are predominantly capacity limited rather than flow-limited this may explain the lack of influence of liver failure among ranolazine users. Even though ranolazine undergoes extensive hepatic metabolism by cytochrome P450, however, ranolazine is primarily renally excreted (75%), thus the presence of chronic liver failure does not affect ranolazine pharmacokinetics16.
Currently, there are three different extended-release doses of ranolazine (375 mg, 500 mg and 750 mg). In 2008, the European Medicines Agency advised 375 mg twice daily as a starting dose of ranolazine and 750 mg twice daily as a maximum dose, while in 2006, the Food and Drug Administration approved 500 mg and 1,000 mg as minimum and maximum doses, respectively.17,18
Under normal conditions, most of the sodium (Na) channels are only temporarily open and rapidly become inactive, resulting in peak Na currents. Under abnormal conditions, Na channel closure is slowed and results in an increase in intracellular Na+.19 An increase in Na+ concentration reverses the direction of the Na+/calcium (Ca)2+ exchange mode, contributing to an excess of Ca2+ in the cell. Increased diastolic Ca2+ interferes with relaxation leading to diastolic dysfunction.19 By inhibiting slow Na currents (Ina), ranolazine is expected to prevent (or reduce) sodium accumulation in myocytes, increase calcium extrusion through Na+/Ca2+ exchange and thereby promote relaxation of the myocardium.20
The pharmacodynamics played by ranolazine is related to the pathophysiology of HF patients with left ventricular relaxation dysfunction (which is a hallmark of HFpEF). One clinical study demonstrated a significant reduction in left ventricular end-diastolic pressure (LVEDP) 30 minutes after IV administration of ranolazine, which was not found in the placebo group.21 Another study found no significant difference in left ventricular end-diastolic volume (LVEDV) between groups.22 Controversial results between these studies may be influenced by the characteristics and quality of each study. The authors were motivated to carry out this meta-analysis as there has not been a systematic review that summarises the published evidence regarding the effectiveness and side-effects of ranolazine before now and there is currently no definitive treatment that has succeeded in significantly improving the quality of life and improving the prognosis of HFpEF patients.
Methods
This systematic review was based on the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines by Cochrane, with the aim of assessing the efficacy of using ranolazine in patients with HF (Supplementary Material Figure 1).
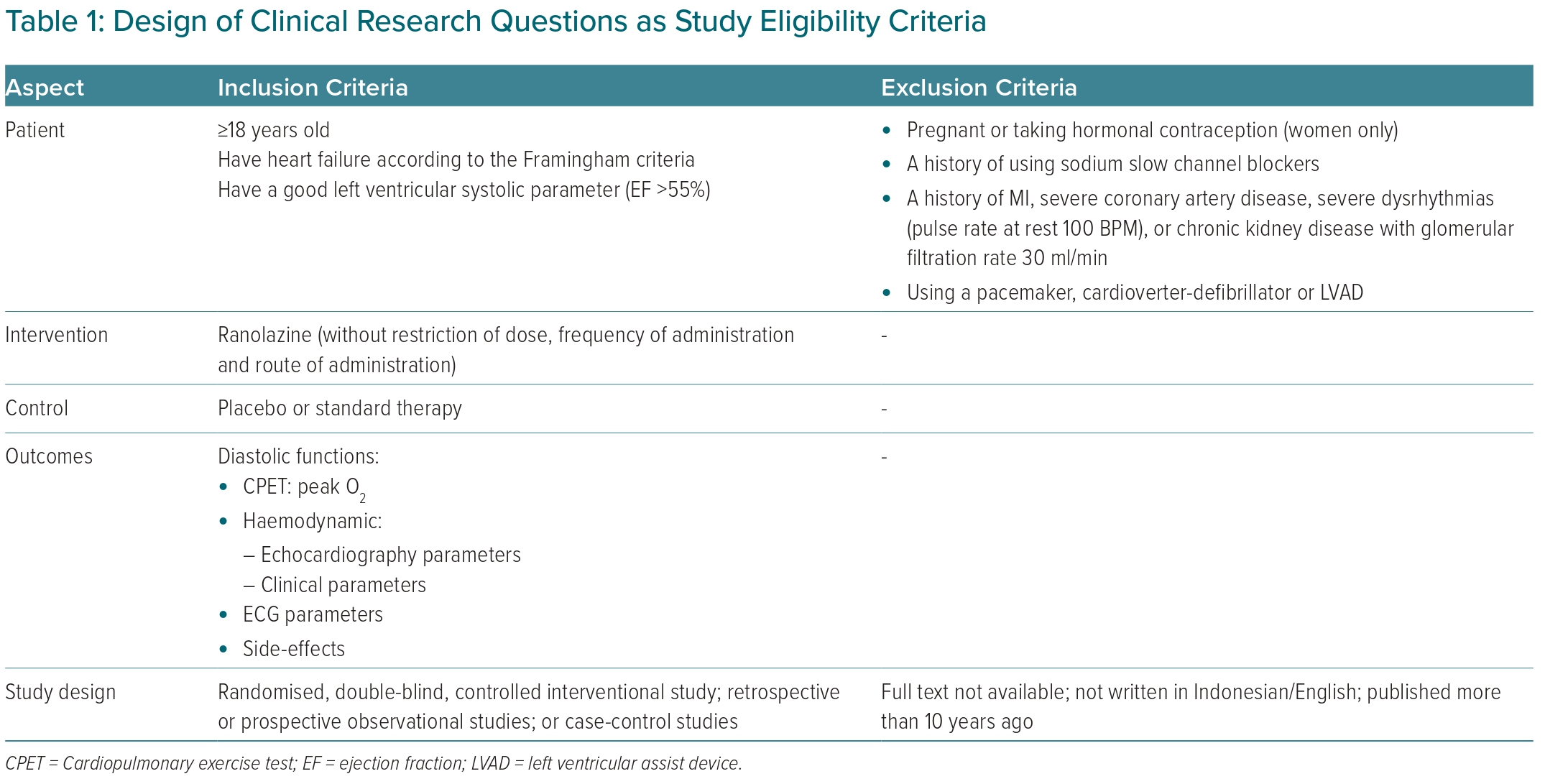
Eligibility Criteria
We included all interventional and observational studies with the inclusion and exclusion criteria listed in Table 1. We also excluded studies that included systematic reviews or meta-analyses, literature reviews, case reports, case series, editorial letters, animal studies and/or unpublished studies that are in the process of peer review. The assessment of the eligibility of the studies was carried out independently by each author. The selection of studies at this stage was done by looking at the title and abstract. Any differences of opinion regarding the eligibility of the study were resolved by discussion.
Literature Search
We conducted a literature search using four databases – MEDLINE, EBSCO, Cochrane Central Register of Controlled Trials (CENTRAL) and ProQuest – with specific keywords customised to the specifications of each search engine. The use of MeSH terminology, as recommended by the Cochrane Handbook 2008, was applied to the literature search.23 We also traced the references of each study to expand the scope of the search.
Study Selection
All relevant articles were entered into our database and viewed for duplication using the Endnote application version x9 for Macintosh. All authors carried out an independent eligibility assessment of all studies based on the full text. Any discrepancies that occured were resolved by discussion or voting.
Data Extraction and Management
All authors extracted data independently. We developed a data extraction sheet referring to the Cochrane Consumers and Communication Review Group.24 Some of the data we extracted included study design, age, number of participants, comorbidities, type of intervention and the aforementioned outcomes.
This systematic review compared the safety and effectiveness of ranolazine in HF with preserved ejection fraction patients against primary (haemodynamic) and secondary clinical outcomes (adverse effects, cardiovascular events, rehospitalisation, quality of life and mortality). Most of the data obtained are in the form of numbers so we used the mean difference of each study to be processed in the meta-analysis. We used a 2x2 format to assess mortality, adverse effect and cardiovascular events, resulting in the RR of each study. We compiled the overall outcomes of each study by weight, using the Review Manager 5 application. Studies with high heterogeneity were analysed using the DerSimonian and Laird random-effects model. A p-value ≤0.05 was considered significant.
Study Quality Assessment
The bias quality assessment was carried out by two independent reviewers (A and B). If there was a difference in the results of the bias assessment, the senior reviewer (C) made the final decision. The risk of bias in this study was assessed using the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool and the revised Cochrane Risk-of-Bias (RoB) tool for randomised trials. The RoB instrument was used to assess the quality of randomised interventional studies with and without blinding.25 The Robins-I tool can be used to assess the risk of bias in interventional studies without randomisation in accordance with the Cochrane recommendations. The Robins-I tool uses the terminology of low, moderate and serious in the risk stratification of the assessed study bias.25
Results
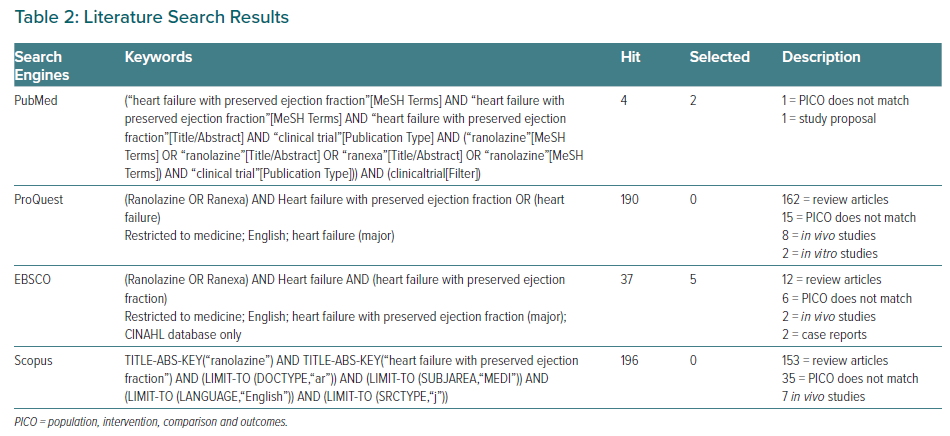
Literature Search
The literature search was conducted using four electronic databases (Table 2) using specific keywords that were customised to the specifications of each search engine. In the initial stage (first hit), we obtained 427 articles with a total of 47 duplicate articles. Overall, we screened the titles and abstracts of 381 articles. A total of 373 articles were excluded for several reasons, such as as irrelevant to the main subjects, outcome evaluation methods were different, language limitation, and the designed comparison were different from the protocol. The eight studies that passed the screening stage had a research design that was in accordance with the population, intervention, comparison and outcomes (PICO) defined in this study, and was analysed in the systematic review stage.
Study Characteristics
This systematic review included eight studies with a randomised controlled trial interventional design. There was some variation in the population included in each study. Two of the studies only included HF patients regardless of comorbidities.21,22 Three studies included patients with ischaemic heart disease with HF syndrome according to Framingham criteria.26–28 One study targeted type 2 diabetes patients with HF syndrome.29,30 Another study recruited patients with hypertrophic cardiomyopathy with a left ventricular thickness of 15 mm.31 A more detailed description of study characteristics is attached in Supplementary Material Table 1.
Bias Risk Assessment
The risk assessment of bias was conducted using the revised Cochrane RoB tool for randomised trials. Blinding of personnel was conducted in the studies of Chaitman et al., Minotti et al. and Shah et al.17,27,29,30 In addition, it is not clear whether the regimen given in the Minotti et al. study was known to patients or not.30 The other five studies had a low risk of bias. A summary of the risk assessment of bias is attached in Supplementary Material Figure 2.
Side-effects
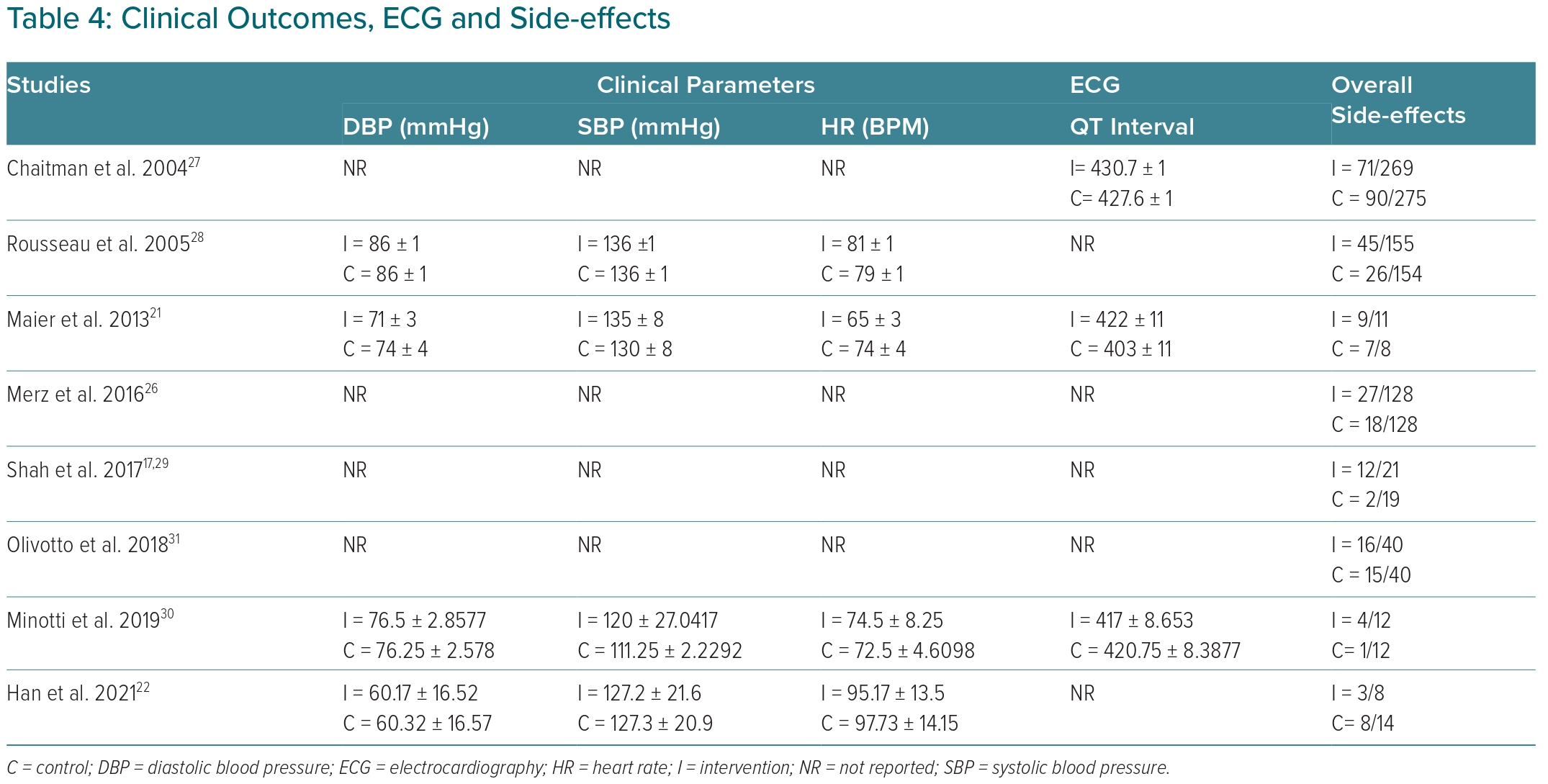
We found substantial heterogeneity (I2 65%) for side-effect analysis. All studies reported the incidence of side-effects using ranolazine, including dyspepsia, nausea, constipation, headache and palpitations. Most studies reported that the incidence of side-effects was more in the ranolazine group. However, we did not find a significant difference in the incidence of adverse events between groups. The relative risk of the ranolazine group for experiencing side-effects was 1.21 (95% CI [0.86–1.69]).
Cardiopulmonary Exercise Test Parameters
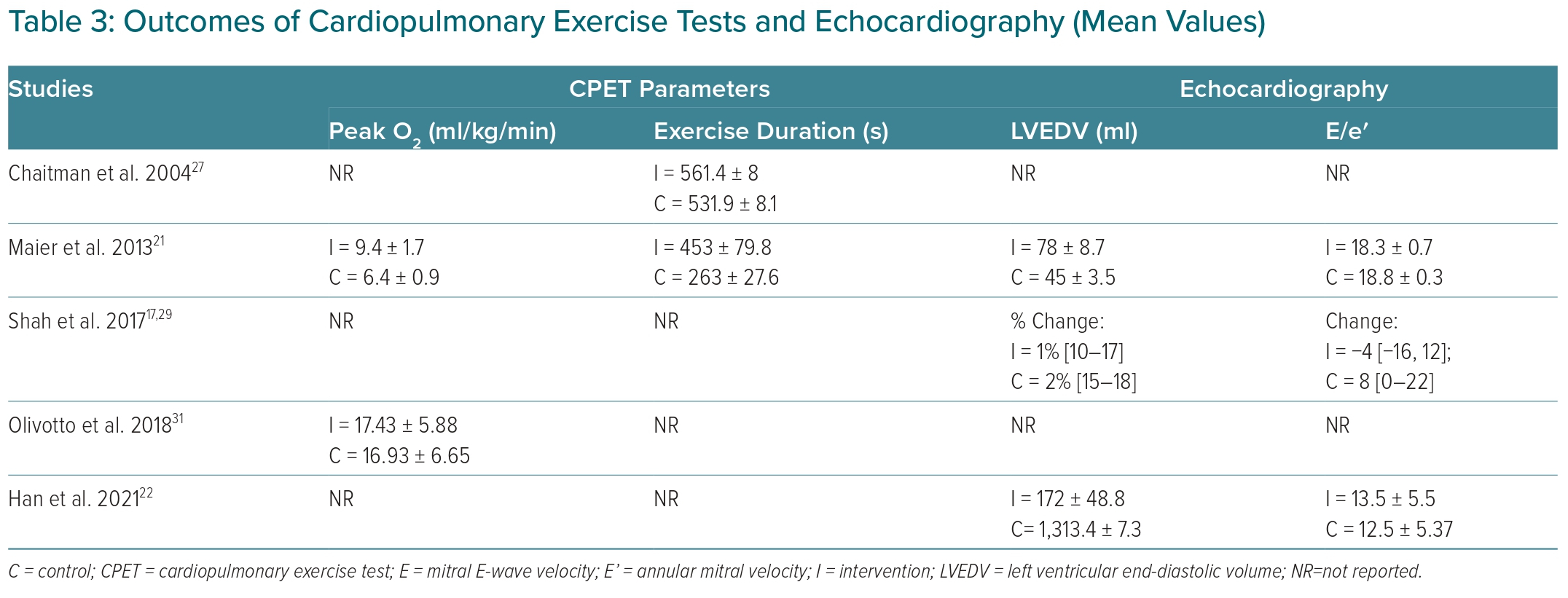
The results of a cardiopulmonary exercise test (CPET) can identify peak O2 and exercise duration. Maier et al. found that peak O2 at 14 days of ranolazine administration was 9.4 ±1.7 ml/kg/min, relatively higher than the baseline data of 8.7 ± 1.3 ml/kg/min, while Olivotto et al. reported peak O2 values that were relatively higher in the ranolazine group (17.43 ± 5.88 versus 16.93 ± 6.55 ml/kg/min).21,31 However, there was no significant difference in peak O2 (p=0.09) between ranolazine and the control group. Substantial heterogeneity was observed in CPET parameters analysis (I2 >63%; Table 3).
Exercise Duration
Two studies were included in the exercise duration analysis. Maier et al. reported that the trend was for the duration of exercise to increase to 453 ± 79.8 seconds from the initial (baseline) 398 ± 168.8 seconds.21 On the other hand, Chaitman et al. reported the exercise duration trend to increase in the ranolazine group (561 ± 8 versus 532 ± 8.1 seconds).27 Based on the results of the forest plot using the DerSimonian and Laird random-effects model (Supplementary Material Figure 3). Even though there was a trend for exercise duration to increase in the ranolazine group, there was no statistical difference between the ranolazine and control group (p=0.18). Substantial heterogeneity (I2: 97%) was observed in the analysis.
Haemodynamic Parameters
In this analysis, we found all parameters were substantially heterogenic (diastolic blood pressure (DBP) I2: 64%; systolic blood pressure (SBP) I2: 99% and heart rate (HR) I2: 94%). We did not find any significant difference between the ranolazine and placebo groups in the DBP (p=0.78), SBP (p=0.26) and HR (p=0.48) parameters (Supplementary Material Figure 4).
Echocardiography
Evaluation of cardiac diastolic function through echocardiography was performed based on the value of the left ventricular end-diastolic volume (LVEDV) and the ratio of the mitral E-wave velocity (E) to the mitral annular velocity (E′). These two parameters were significantly different between the ranolazine and placebo groups. We found that the ranolazine group had a significantly higher mean LVEDV with a mean difference between the two groups of 33.34 (95% CI [27.18–39.50]) and p<0.001, using the Mantel-Haenszel fixed-effect model. The E/E′ value was also found to be significantly lower in the ranolazine group, with a mean difference of 0.45 (95% CI [27.18–39.50]) and p=0.05 (Supplementary Material Figure 5). Both parameters had low heterogeneity results (LVEDV I2= 0% and E/E′ I2= 32%).
ECG Parameters
Three of the studies report the outcome of the QTc interval as a parameter of the effectiveness of ranolazine therapy compared to placebo. Studies conducted by Chaitman et al. and Maier et al. showed a trend to increase QTc interval, while Minotti et al. reported a trend to decrease QTc interval in the ranolazine group compared to the control group.21,27,30 However, we found there was no statistical difference (I2:86%, p=0.27) between ranolazine and the control group regarding QTc interval. (Table 4).
Discussion
This meta-analysis found that ranolazine has good effectiveness in the management of HfpEF, especially in maintaining or improving diastolic dysfunction. To the best of the authors’ knowledge, there has not been a meta-analysis with the same study design and research questions as this meta-analysis, thus, the value of originality and novelty of this study is considered quite good. The echocardiography parameter, which is the main outcome in evaluating the patient’s diastolic function, also uses a fixed effect model calculation, as the patient characteristics were relatively homogeneous. This leads to a minimal risk of bias due to differences in the baseline characteristics of patients in the study.
This systematic review attempts to combine the findings reported by various studies regarding the effectiveness of ranolazine in the management of patients with HF, as well as the side-effects that occur during the administration of drugs acting on this INa channel. There were eight randomised controlled trials of moderate-to-good quality included in this meta-analysis. Mortality and hospital admission outcomes were not reported in all studies. Alternatively, we quantified the incidence of adverse events, CPET parameters, echocardiography, ECG and clinical measures (blood pressure and heart rate) as the basis for assessing the effectiveness of using ranolazine. CPET measures heart and lung activity when given an aerobic exercise load. Maier et al. reported that peak O2 and duration of exercise were noted to be significantly higher in the ranolazine group than in the placebo, with values of 9.4 ± 1.7 ml versus 6.4 ± 0.9 ml (peak O2) and 453 ± 79.8 seconds versus 263 ± 27.6 seconds, respectively.21 Olivotto et al. found peak O2 values that were not significantly different between groups.31 The combined findings of all studies showed that there was no significant difference in CPET outcomes between the ranolazine and placebo groups. Although no significant differences were found, both in peak O2 outcomes and exercise duration, the ranolazine group scores were relatively higher than the placebo group. The absence of this significant difference could be due to the short duration of ranolazine administration, while endurance generally only changed after at least 10 weeks.32
We found that the ranolazine group had significantly higher LVEDV values than the placebo group. Patients with HFpEF exhibit decreased left ventricular active relaxation due to a combination of impaired cardiac energy, increased ventricular wall stiffness and vasculo-ventricular mismatch.33 This impaired diastolic filling causes increases in LVEDP and pulmonary artery pressure. As pressure is inversely proportional to volume, LVEDV will decrease in patients with HFpEF.34 An increase in LVEDV in the ranolazine group indicates that there is an improvement in diastolic function after administration of ranolazine. Ranolazine blocks the current of sodium and potassium ion channels. Late-phase inhibition of inward sodium flow during cardiac repolarisation has been identified to play a role in HF. In pathological conditions, there is an increase in sodium-calcium exchange due to an increase in late-phase sodium influx activity, which ultimately leads to an increase in cytosolic calcium concentrations. Excess intracellular calcium is believed to be important for the mechanism of decreased left ventricular relaxation caused by ischaemia and reperfusion. An increase in left ventricular diastolic wall pressure impairs myocardial blood flow further. In addition, excess calcium has an adverse effect on the electrical activity of the myocardium leading to ventricular tachycardia.35 This pathophysiological cascade of HF is ameliorated by ranolazine targeting the slow INa channel.36 The finding that the ranolazine group had a significantly higher LVEDV than placebo is in line with ranolazine’s pharmacodynamic theory, which is the basic idea of using this drug in patients with HFpEF. In addition, ranolazine also has a mild coronary dilatation effect that may contribute to improved diastolic parameters in HFpEF and cardiac ischaemia. Therefore, ranolazine was associated with a decrease in left ventricular relaxation.18,39,40,41,42
Apart from higher LVEDV, other parameters of diastolic dysfunction (E/E′) were also found to be lower in the ranolazine group with a mean difference of 0.45 (95% CI [27.18–39.50]). This further supports the idea that ranolazine has an effect in improving diastolic function in HF patients in the studies used in this meta-analysis.
Ranolazine, a piperazine derivative, has good metabolic properties and does not affect heart rate or blood pressure.18 This theory is in line with our findings in this meta-analysis in which systolic and diastolic blood pressures were not significantly different between ranolazine and placebo groups. Heart rate frequency was also not found to be significantly different between groups. In addition, we documented relatively higher diastolic blood pressure values in the ranolazine group. Low diastolic blood pressure is actually a poor prognostic factor related to patient survival.37 The ECG parameter – QT interval assessment – aims to evaluate the increase in the rate of ventricular repolarisation due to inhibition of the cardiac INa channel. Ranolazine plays a role in blocking this channel, thereby increasing calcium efflux, which in turn triggers ventricular repolarisation.38 In this meta-analysis, we did not find QT interval shortening in any of the studies that looked at this outcome. We also found no significant difference in QT interval between the ranolazine group and the placebo group. In addition to concerns related to shortening the QT interval, some side-effects of concern include palpitations, syncope, nausea and various other clinical complaints. In this study, we didn’t find any difference in the proportion of patients who experienced any side-effects between groups. Patients on ranolazine therapy had a relative risk of adverse events of 1.21 (95% CI [0.86–1.69]).
However, there are some things that need to be underlined regarding our findings. First, the dose and duration of ranolazine administration varied between studies. One even used an IV preparation as a loading dose, while no other study used this regimen. Second, this study did not consider comorbidities as inclusion or exclusion criteria. Finally, two studies with moderate risk of bias were included in the meta-analysis. That was felt to interfere with the final interpretation of this meta-analysis.
Conclusion
Ranolazine may have good effects on patients who have HFpEF with a higher LVEDV value and reduced E/e′ as a marker of diastolic function compared to the standard treatment alone. It is particularly safe as ranolazine doesn’t affect blood pressure, heart rate and rate of ventricular repolarisation (shortening of the QT interval).
Click here to view Supplementary Material.
Clinical Perspective
- To date, our understanding regarding the pathophysiology of heart failure with preserved ejection fraction (HfpEF) is incomplete and despite intensive efforts, optimal therapy remains uncertain as most trials to date have had negative outcomes.
- Ranolazine has a good efficacy to improve diastolic performance among patients with HFpEF.
- There is no significant improvement in cardiopulmonary exercise test performance found in the form of peak O2 and exercise duration when taking ranolazine.