AF is the most common sustained arrhythmia encountered in clinical practice. It is a chronic and progressive disorder initially characterised by exacerbations and remissions leading to reductions in quality of life (QOL) with AF comparable to that observed in patients with chronic heart failure or receiving chronic haemodialysis. Furthermore, AF is associated with an increased risk of stroke and heart failure, leading to reduced overall survival.1–3 The contemporary goals of AF management are improving arrhythmia-related symptoms and QOL, as well as reducing the morbidity and healthcare utilisation associated with AF.4
Without treatment, AF will recur in up to 75% of patients within a year of their index episode.5–7 Antiarrhythmic drugs (AADs) have been proven to be more effective than placebo for the maintenance of sinus rhythm. However, they have only modest efficacy at maintaining sinus rhythm and are associated with significant cardiac adverse effects (e.g. bradydysrhythmia, negative inotropy and pro-arrhythmia) and non-cardiac adverse effects (e.g. end-organ toxicity), including increased mortality with the long-term use of amiodarone and sotalol (OR 2.73; 95% CI [1.00–7.41]; p=0.049 and OR 4.32; 95% CI [1.59–11.70]; p=0.013, respectively).8,9
Over the past 20 years, catheter ablation, which is centred on the electrical isolation of triggering foci within the pulmonary veins, has been shown to be superior to AAD therapy when AADs have been ineffective, or are contraindicated or poorly tolerated.10
While it has been postulated that early intervention may provide significant benefits, i.e. catheter ablation as an initial therapy prior to AADs, we cannot extrapolate the evidence supporting the role of catheter ablation as a second-line therapy as these trials were performed in patients who had already failed pharmacotherapy, thus weighting the benefit towards catheter ablation.
AF Ablation as a First-line Therapy
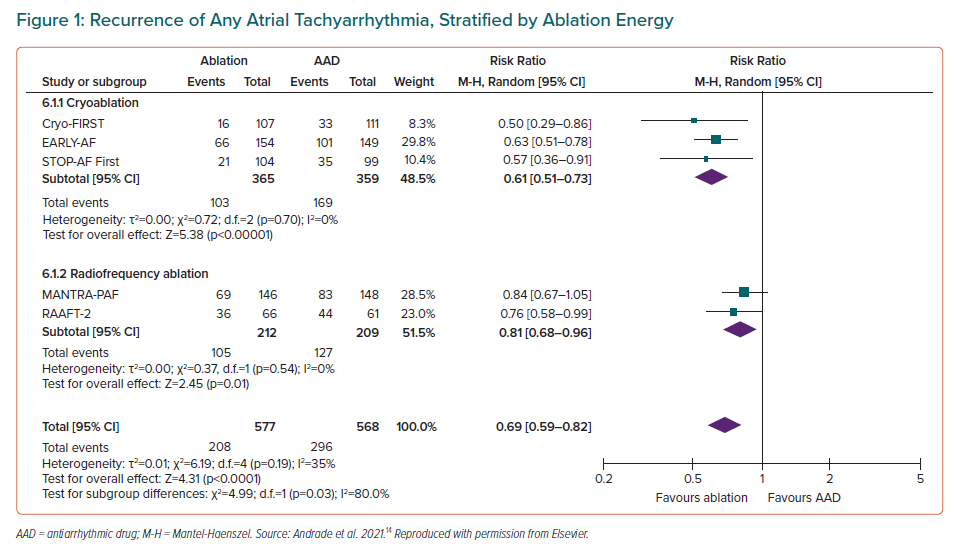
Studies have attempted to answer whether a population may exist whereby the effectiveness of a catheter ablation procedure would be sufficiently high, and the risks sufficiently low, that it would be appropriate to offer ablation as an initial therapy. Prior trials of first-line radiofrequency (RF) catheter ablation have been primarily limited by their relatively small sample size, high rates of cross-over from AADs to ablation and the use of intermittent non-invasive rhythm monitoring, which has limited the ability to detect a difference between treatment groups.11–13 In aggregate, the three randomised studies of first-line RF ablation reported a relatively low absolute success rates (46–53% freedom from atrial tachyarrhythmia in the ablation arm versus 28–44% in the AAD arm), and consequently low relative benefit with first-line RF ablation (RR 0.81 for any arrhythmia; 95% CI [0.68–0.96]; p=0.01; Figure 1, and RR 0.62 for symptomatic arrhythmia; 95% CI [0.38–1.01]; p=0.06; Figure 2).14
Guideline Recommendations
Given the relatively low success rate, the lack of procedural standardisation, and the inconsistent procedural endpoints, the major North American and European guidelines provide only a conditional recommendation for catheter ablation as first-line therapy.15–17 Specifically, reserving it for rare individual circumstances, or highly-selected patients with symptomatic paroxysmal (Class IIA in European Society of Cardiology [ESC] and American Heart Association [AHA]/American College of Cardiology [ACC]/Heart Rhythm Society [HRS] guidelines) or persistent AF (Class IIB in ESC and AHA/ACC/HRS guidelines, with the ESC suggesting that first-line ablation be restricted to those without major risk factors for AF recurrence).15,16 Likewise, the Canadian Cardiovascular Society (CCS) guidelines provide a weak recommendation for first-line catheter ablation in select patients with symptomatic AF. However, in contrast to the ESC and ACC/AHA/HRS, the CCS makes no distinction between those with paroxysmal or persistent AF.17
The Early Aggressive Invasive Intervention for AF Trial: EARLY-AF
Design
The emergence of single-shot AF ablation toolsets has led to renewed interest in determining whether first-line catheter ablation may improve outcomes. In contrast to point-by-point RF ablation, single-shot AF ablation toolsets has enabled enhanced procedural standardisation, ensuring consistent outcomes with low rates of complications.
Despite varying operator skillsets, cryoballoon ablation has been shown to be associated with a high acute procedural success and long-term freedom from recurrent AF, with low rates of serious complications.18,19 This balance of generalisability, safety and efficacy suggests that cryoballoon ablation may be a preferred toolset for initial (e.g. first-line) ablation.
The EARLY-AF trial sought to evaluate the role of initial cryoballoon ablation versus initial AAD therapy as the first treatment of AF in AAD-naïve patients.20 The study was a multicentre parallel-group, single-blinded randomised clinical trial, with blinded end-point ascertainment conducted at 18 clinical centres in Canada. The trial enrolled 303 patients, randomising 154 to undergo initial cryoballoon ablation and 149 to receive initial AAD therapy.9
Patients enrolled in the study were relatively young and free of significant comorbidities. At baseline, the mean age was 58.6 years, 29.4% were female, 37% had hypertension, 9% had heart failure and 3% had previous stroke or transient ischaemic attack. Enrolled patients had been diagnosed with AF a median of 1 year prior to enrolment. Patients were highly symptomatic (mean AF effect on quality of life [AFEQT] score of 59.4) and were experiencing a median of three symptomatic AF episodes per month (interquartile range [IQR] 1–10).
Patients randomised to cryoballoon ablation underwent circumferential pulmonary vein isolation using a second-generation cryoballoon, with the procedure endpoint of bidirectional conduction block. Complete pulmonary vein isolation was confirmed in all patients, with a median left atrial time of 74 minutes (IQR 56–94) and procedure duration of 106 minutes (IQR 89–131), including a mandatory 20-minute observation period.
Patients randomised to AAD therapy had their AADs aggressively optimised using standardised titration protocols.9,20 Class IC sodium-channel blockers were the most frequently prescribed agents, with the most frequently prescribed AAD being flecainide (used in 83.2% of patients) at a median daily dose of 200 mg (IQR 125–250). Multiple AAD trials were required in 46 patients (30.9%). All patients in the AAD group exited the 90-day treatment optimisation period on a therapeutic dose of AAD.
Primary and Secondary Outcomes
All patients in the EARLY-AF trial received an implantable cardiac monitor for continuous rhythm monitoring, with all arrhythmia events undergoing independent adjudication by a committee blinded to treatment allocation.
The primary outcome was the first recurrence of any atrial tachyarrhythmia, defined as AF, atrial flutter or atrial tachycardia lasting ≥30 seconds between 91 and 365 days after treatment initiation (i.e. catheter ablation or AAD initiation).
Secondary outcomes including the first recurrence of symptomatic atrial tachyarrhythmia between 91 and 365 days, AF burden, disease-specific and generic QOL, healthcare utilisation (cardioversion, emergency department visit, and hospitalisation, alone and in aggregate) and adverse events. Serious adverse events were defined as those causing death or functional disability, warranting intervention, or resulting in or prolonging hospitalisation for more than 24 hours.
Rhythm Outcomes
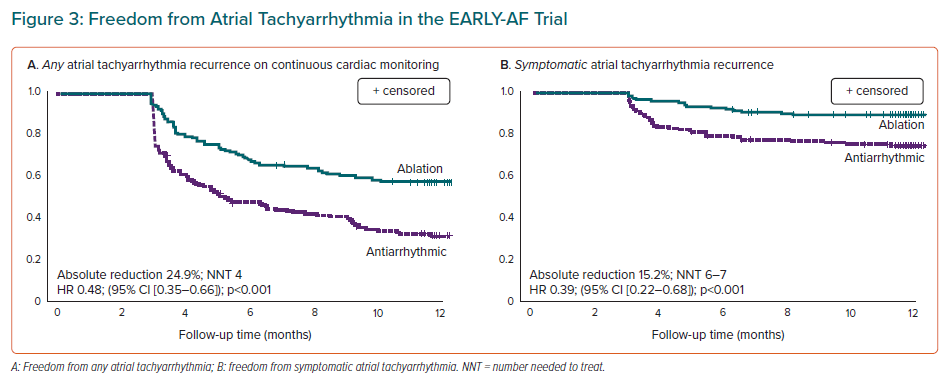
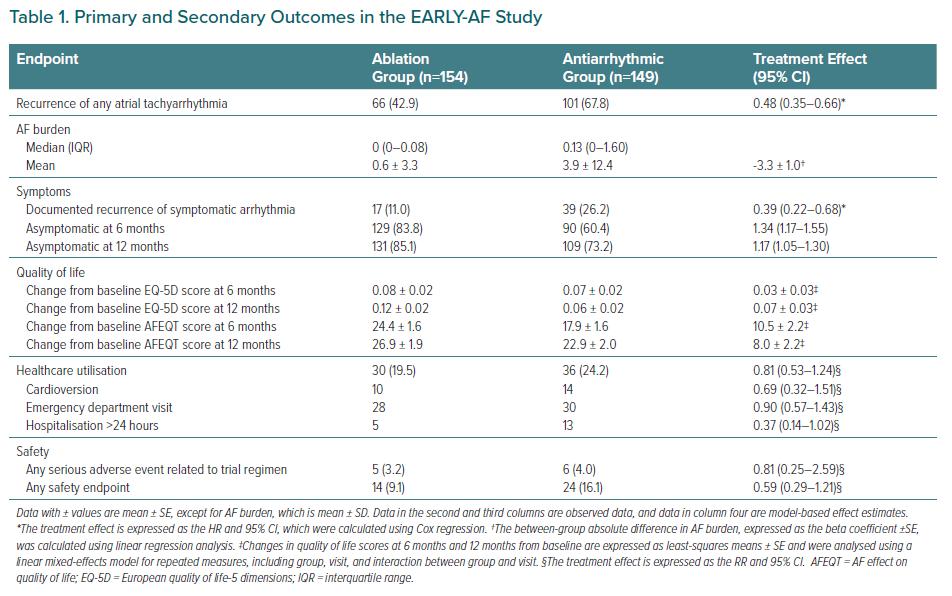
Documented recurrence of any atrial tachyarrhythmia occurred in 42.9% of patients randomised to cryoballoon ablation and 67.8% of patients randomised to AADs within 1 year following treatment initiation (HR 0.48; 95% CI [0.35–0.66]; p<0.001; Figure 3A).9
Symptomatic atrial tachyarrhythmias occurred in 11.0% of patients randomised to ablation, and 26.2% of patients randomised to AADs at 1 year (HR 0.39; 95% CI [0.22–0.68]; Figure 3B). These results correspond to a number needed to treat (NNT) of four to prevent one asymptomatic atrial tachyarrhythmia recurrence and an NNT of seven to prevent one symptomatic atrial tachyarrhythmia recurrence.
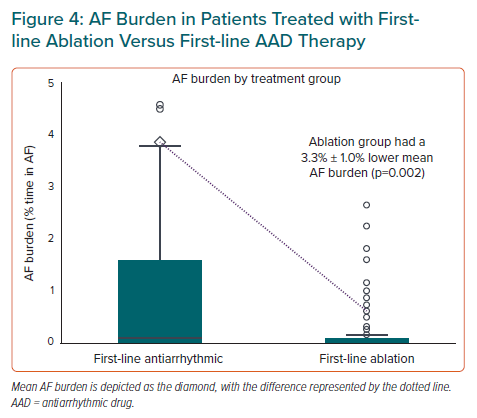
The absolute difference in mean AF burden between patients randomised to ablation and patients randomised to AADs was 3.3% ± 1.0% (Figure 4). This difference corresponded to the equivalent of 1 day less of AF per month for patients randomised to ablation.
Quality of Life Outcomes
The change in disease-specific and generic QOL scores at 1 year was significantly improved in both groups. For the disease-specific AFEQT questionnaire, those randomised to ablation attained a 26.9 ± 1.9 points improvement from baseline at 1 year, with those randomised to AADs achieving a 22.9 ± 2.0 points improvement from baseline (Table 1). For the generic European Quality of Life-5 Dimensions (EQ-5D) survey, the improvement in QOL score was 0.12 ± 0.02 and 0.06 ± 0.02 for the ablation and antiarrhythmic groups, respectively. At 1 year the mean treatment effect (the difference between randomised groups) was 8.0 ± 2.2 points in favour of ablation for the AFEQT score and 0.07 ± 0.03 points for the EQ-5D score. These between-group differences exceeded the minimally clinically relevant difference (e.g. 5 points on AFEQT score and 0.03 points on EQ-5D score).21
Healthcare Utilisation
While the overall outcome of healthcare utilisation was not significantly different between randomised groups, the first-line ablation group had a numerical reduction in the individual healthcare utilisation events (cardioversion, emergency department visits and hospitalisation; Table 1).
Safety Outcomes
Serious adverse events occurred in 3.2% of patients randomised to ablation (three self-limited phrenic nerve palsies and two pacemaker implantations for bradydysrhythmia) and 4.0% of patients randomised to AADs (three wide-complex dysrhythmias, two pacemaker implantations for bradydysrhythmia and one heart failure exacerbation), with no significant difference between groups (Table 1). Any safety endpoint was observed in 9.1% of patients randomised to ablation and 16.1% of patients randomised to AADs, with no significant difference between groups.
Implications
The design of the EARLY-AF trial included several unique features in an effort to address the limitations of the previous first-line ablation studies. Specifically, while intermittent non-invasive rhythm monitoring is the most widely used method of ascertaining arrhythmia recurrence, it lacks sensitivity in detecting sporadic arrhythmias (e.g. paroxysmal AF), which leads to under-detection of recurrences. This under-detection leads to inflated estimates of treatment success and introduces misclassification errors that impact the accuracy and precision of comparative risk estimates. Instead, the EARLY-AF trial relied on implantable loop recorders, which facilitated precise determination of the presence and timing of arrhythmia recurrence, as well as accurately quantified enumerable outcomes such as arrhythmia burden. Second, in an effort to ensure that the treatment comparison was robust, the dose of AADs in the pharmacotherapy group was aggressively up-titrated using standardised protocols over a 3-month treatment optimisation period with a goal of complete suppression of AF on loop recorder monitoring.20 Third, the trial employed pre-specified protocols, including the establishment of an independent committee, in order to ensure that no patient crossed over between randomised groups prior to the occurrence of a primary endpoint event.
Results in Context
The EAST-AFNET 4 trial recently tested an early rhythm-control strategy in patients with newly-diagnosed AF (enrolled median 36 days after AF diagnosis). This trial predominantly employed pharmacological rhythm control, demonstrating that early rhythm control significantly reduced the composite primary outcome of cardiovascular death, stroke, and hospitalisation for worsening heart failure and acute coronary syndrome by 21% (from 5.0% per year to 3.9% per year) but increased serious adverse events related to AAD therapy (4.9% versus 1.4% in the usual care group).22 Effectively, the EAST-AFNET 4 trial has provided convincing evidence of the benefits of aggressively pursuing sinus rhythm maintenance, answering the question of ‘why’ we might pursue rhythm control for patients with newly-diagnosed AF.
Instead, the EARLY-AF trial focuses on the question of ‘how’ rhythm control can be effectively achieved in highly symptomatic patients with treatment-naïve AF. While the majority of patients enrolled in the EAST-AFNET 4 trial were treated with AAD therapy (only 8.0% and 19.4% undergoing AF ablation at baseline and 2 years of follow-up), the EARLY-AF trial demonstrated that an initial cryoballoon ablation approach was superior to AADs for the outcomes of atrial tachyarrhythmia recurrence, arrhythmia burden and QOL. These findings are relevant to patients, providers, and healthcare systems when considering the initial treatment choice for rhythm-control therapy.
In addition to EARLY-AF, two other multicentre randomised trials have compared initial cryoballoon ablation to AADs in patients with symptomatic, treatment-naïve paroxysmal AF: the Cryo-FIRST trial and the STOP-AF First trial.23,24 In total these three randomised trials included 724 patients in their intention-to-treat or modified intention-to-treat populations.9,23,24 In pooled analysis, initial cryoballoon ablation significantly reduced the recurrence of atrial tachyarrhythmia when compared with first-line AAD therapy (risk ratio 0.61; 95% CI [0.51–0.73]), with a NNT of seven (weighted absolute risk reduction of 19%; Figure 3B).14 In addition, patients treated with initial cryoballoon ablation were significantly more likely to be free of symptoms at 12 months of follow-up (80% versus 68%, risk ratio 1.16; 95% CI [1.05–1.28]). While both treatment groups improved from baseline, initial cryoballoon ablation was associated with a significantly greater improvement in QOL (mean between-group difference of 8.46-point in the disease-specific AFEQT; 95% CI [5.86–11.06]). While no study was individually powered for healthcare utilisation endpoints, pooled analysis demonstrates that significantly fewer patients randomised to first-line cryoballoon ablation experienced the composite healthcare utilisation outcome (risk ratio 0.71; 95% CI [0.56–0.90]), with a NNT of 12 to prevent one healthcare utilisation endpoint (weighted absolute risk reduction of 9%). This was driven by a significant reduction in all-cause hospitalisation (risk ratio 0.38; 95% CI [0.23–0.63]; weighted absolute risk reduction of 12%), with non-significant reductions in emergency department visits (risk ratio 0.78; 95% CI [0.50–1.20]) and cardioversions (risk ratio 0.60; 95% CI [0.31–1.18]). Despite the invasive nature of an AF ablation procedure, the risk of serious treatment-related adverse events was comparable between initial cryoballoon catheter ablation and initial AAD therapy (risk ratio 0.74; 95% CI [0.35–1.56]), with ablation having a lower risk of any adverse event (risk ratio 0.70; 95% CI [0.54–0.89]).
Unanswered Questions and Extrapolation of Results
Despite the wealth of data from these studies, several unanswered questions remain. Specifically, first if these results are generalisable to other ablation energy sources or to patients with more advanced forms of AF, and second if early ablation results in beneficial effects on progression to more persistent forms of AF.
Regarding generalisability, recent randomised clinical trials have observed similar outcomes for patients with AAD-refractory AF treated with cryoballoon ablation and contact-force RF ablation.25 However, previous studies of first-line RF ablation failed to demonstrate a clinically meaningful difference in arrhythmia outcomes, QOL improvement, and healthcare utilisation.11–13 It is possible that the difference in results may be related to the greater heterogeneity in outcomes observed after RF ablation, where annual procedure volume has been significantly associated with efficacy.26 Conversely, cryoballoon ablation outcomes have not been not significantly associated with operator and centre volume, suggesting that the ‘single-shot’ pulmonary vein isolation produced by cryoballoon allows a variety of operators to achieve more consistent and reproducible procedural outcomes. As such, in the absence of comparative trials, it may be reasonable to extrapolate the results of these first-line cryoballoon studies to RF ablation performed in high-volume centres, particularly when guided by standardised workflow (e.g. the CLOSE protocol). However further study is required to determine whether the results of first-line catheter ablation are comparable in lower volume RF ablation centres.
Moreover, the majority of patients enrolled in these studies were young, relatively healthy, and predominantly afflicted with paroxysmal AF. Strictly speaking, it is not known whether the results of these first-line ablation studies can be extrapolated to patients with more advanced (e.g. persistent) forms of AF. However, there is evidence to suggest that these results may be applicable in this population as comparable relative reductions in AF burden observed after ablation of both paroxysmal AF and persistent AF.27
Regarding the second question, it is postulated that intermittent AF episodes result in cumulative electrical and structural atrial remodelling, enabling the progression from paroxysmal to persistent forms of AF. To date, several observational studies have suggested that a shorter time interval between the AF diagnosis and catheter ablation is associated with improved outcomes.28,29 Recently the randomised ATTEST trial observed that ablation performed better than guideline-directed AAD therapy in delaying the progression from paroxysmal to persistent AF.30 Longer-term follow-up of the patients enrolled in the first-line cryoablation studies will provide further insight.
Conclusion
An initial treatment strategy of first-line cryoballoon ablation in patients with treatment-naïve AF was superior to AADs. Compared to AADs, an initial treatment strategy of cryoballoon catheter ablation resulted in greater freedom from atrial tachyarrhythmia recurrence, a superior reduction in AF burden, greater improvement in QOL, and significantly lower subsequent healthcare resource utilisation. These findings are relevant to inform patients, providers and health care systems regarding the choice of initial rhythm-control therapy in patients with treatment-naïve AF.