Chronic thromboembolic pulmonary hypertension (CTEPH) is characterised by fibrothrombotic material mechanically obliterating major pulmonary arteries, resulting in increased pulmonary vascular resistance (PVR), progressive pulmonary hypertension (PH) combined with a microscopic pulmonary vasculopathy, right ventricular (RV) failure and premature death.1,2 The survival of patients with CTEPH in the 1980s was poor.3 However, data from an international registry between 2007 and 2009 reported survival rates of 92%, 75%, and 60% at 1, 3 and 5 years, respectively.4 Data from the most recent European registry recruiting between 2015 and 2016 suggest even better survival.5 Surgical pulmonary endarterectomy (PEA) and balloon pulmonary angioplasty (BPA), in combination with vasodilator drugs, have markedly improved outcomes in CTEPH.4–8 However, recovery is not uniform, and there are patients who cannot undergo mechanical treatments and others who do not respond well to medical treatments. BPA can be performed as a stand-alone procedure, simultaneous with PEA, in sequence with PEA, in combination with medical treatments and in combination with PEA and medical treatments.9 This review focuses on the efficacy and safety outcomes of BPA as they stand today, and reports the state of knowledge regarding sex differences.
Efficacy of Balloon Pulmonary Angioplasty
Historical Outcomes
In 2001, Feinstein et al. described 18 patients with inaccessible or ‘nonsurgical’ CTEPH who were subjected to BPA.10 Reperfusion pulmonary oedema occurred in 11 patients, and 30 day mortality was 5.5%. At a mean follow-up of 36 months, improvements were reported in mean pulmonary arterial pressure (mPAP), New York Heart Association functional class and the 6 minute walk distance (6MWD), and all vessels previously dilated were patent at angiographic reassessment.10 Together, the results indicate that safety issues were compromising efficacy outcomes during the early treatment days.
Chronic Thromboembolic Pulmonary Hypertension and Sex
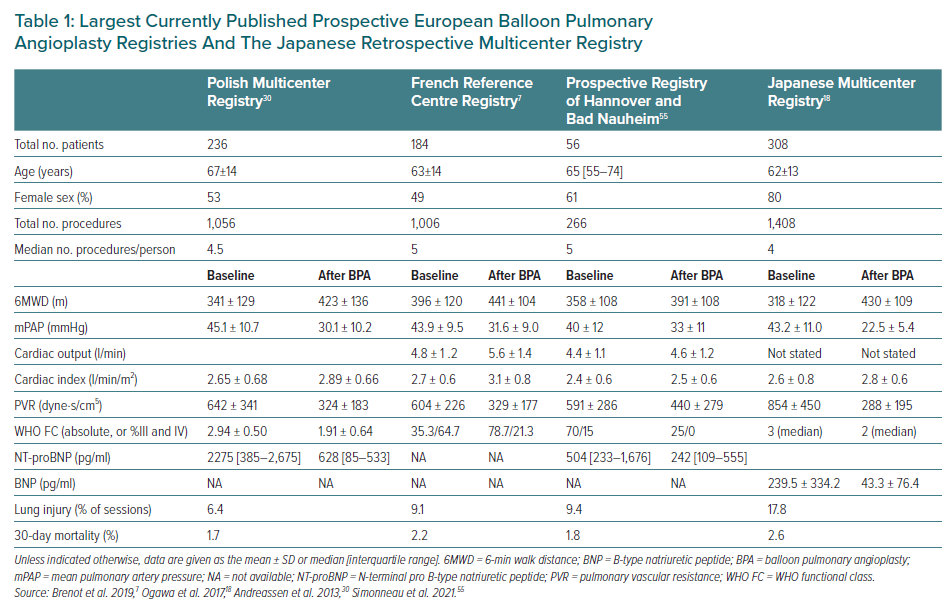
Although in Europe CTEPH is almost equally frequent in men and women in their sixth decade of life, most CTEPH patients in Japan are women.11 This key difference between European and Japanese CTEPH patients is illustrated in BPA databases (Table 1). In the European CTEPH Registry, a majority of females (56%) were classified as inoperable.12 Female CTEPH patients had a lower prevalence of cardiovascular risk factors and were less often exposed to additional cardiac surgery procedures when they underwent PEA and had better long-term survival.12 In Japan, female CTEPH patients are elderly with less deep vein thrombosis, less acute embolic episodes, better cardiac function, lower arterial oxygen tension and more peripheral thrombi and derive less improvement through PEA.11 A systematic sex-specific analysis of BPA outcomes will be performed within the International BPA Registry (NCT03245268).
Outcomes in Japan
Although BPA was abandoned in the US and Europe in 2001, Japanese interventionists refined the technique over the next 10 years, leading to a more cautious stepwise approach, subsequent sessions and the use of 0.014-inch guidewires.After 2012, the efficacy of BPA in improving haemodynamics and exercise capacity was established by case series reported by several groups.13–17 In 2017, the Japanese Multicenter Registry was reported, retrospectively summarising the experience of seven Japanese institutions (Tohoku University, Tokyo University, Kyorin University, Mie University, National Cardiovascular Research Center, Kobe University and National Hospital Organization Okayama Medical Center) between November 2004 and March 2013.18 Overall survival after the final BPA procedure for 249 patients was 98.9% at 1, 2 and 3 years, data that are comparable to survival after PEA.5 In the Japanese Multicenter Registry, approximately 44% of patients were on pulmonary vasodilators at the time of the last haemodynamic assessment after completing BPA.18 The Sendai Center published a separate report of 424 BPA sessions in 77 consecutive patients; between baseline and complete BPA, mPAP changed from 38±10 to 25±6 mmHg, PVR changed from 7.3±3.2 to 3.8±1.0 Wood units, and 6MWD improved from 380±138 to 486±112 m (all p<0.01 mean ± SD).19 In that report, 5-year survival was 98.4%, with no periprocedural deaths, which is similar to the multicentre experience.19 Persistent vasodilator treatment after complete BPA was 68% directly after BPA, and decreased further to 22% beyond 6 months.19 The concurrence of pulmonary vasodilator intake is an important consideration when reporting BPA outcomes. Japanese investigators defined complete BPA as BPA leading to mPAP <25 mmHg at rest, whereas more aggressive goals of reducing residual lesions to fewer than five segments have been suggested as extensive revascularisation by BPA.20 Extensive BPA beyond normalisation of resting pressures led to improved exercise tolerance (i.e. an improvement of approximately 9% in peak VO2 values, from 17.3 to 18.9 ml/kg/min) and better physical quality of life scores.21,22
Overall, haemodynamic results from Japan, as shown in Table 1, appear to be better than in Europe.23 This may be due to intrinsic differences between European and Japanese patients, with European CTEPH patients having higher serum concentrations of C-reactive protein, fibrinogen and myeloperoxidase, and more red thrombus than Japanese CTEPH patients.24 Furthermore, because mean pulmonary arterial wedge pressures are higher in European patients due to concurrent left heart disease, mean pulmonary artery pressures tend to be higher as well. Positive effects on outcomes are accumulating from smaller series.16,25–28
Outcomes in Europe
The European BPA experience was pioneered by Andreasen, still with large equipment and a periprocedural mortality of 10%.29 Since 2013 the Japanese technique was taught across Europe and all large CTEPH centres started their own BPA programs. Data from the first prospective European registries are presented in Table 1 in comparison with data from the Japanese Multicenter Registry. BPA is significantly improving 6MWD, New York Heart Association functional class, biomarkers and haemodymamics. European centres reported significant changes in outcomes, regarding both efficacy and safety, as a consequence of learning curves.7,30–37 More lesions are addressed per session and chronic total occlusions are treated, conferring particular benefit.38–41 A direct comparison between BPA and medical treatments with riociguat in the RACE trial (NCT02634203) illustrated the greater efficacy of BPA (at week 26, geometric mean PVR decreased to 41% of baseline in the BPA group, compared with 68% of baseline in the riociguat group), albeit at the price of procedure-related complications. BPA was associated with more treatment-related serious adverse events (42% versus 9% of patients).42
Functional Improvement After Balloon Pulmonary Angioplasty
In parallel to haemodynamics at rest, right ventricular functional improvements are documented by echocardiography and cardiac magnetic resonance, echocardiographic speckle tracking analysis and phase-contrast MRI.43–48 However, for cardiopulmonary disease, dynamic improvement in exercise capacity is of particular interest. Studies after PEA have shown that despite normalised resting haemodynamics, exercise limitations persist.49,50 Similar observations have been reported after BPA.51 However, one study suggested that the aetiology of persistent exercise limitation may derive from concomitant left heart disease.52 A prospective single centre European study showed that improvements in pulmonary haemodynamics at rest and during exercise, in quality of life and in exercise capacity were observed 6 months after BPA and that WHO functional class improved in 78% of patients.53 Some data suggest that riociguat exerts beneficial effects on haemodynamic responses to exercise in CTEPH patients on top of haemodynamic improvements by BPA.54
Safety of Balloon Pulmonary Angioplasty
The occurrence of complications is proportional to operator experience and can be significantly reduced during a learning curve (e.g. in France, severe lung injury was reduced from 20% to 4% per patient).58
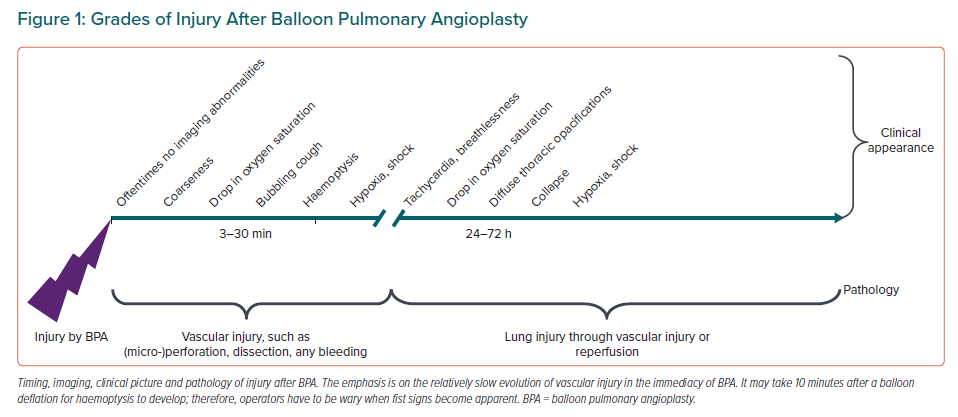
A classification of complications needs to take into account thoracic (Figure 1) and non-thoracic complications. Thoracic complications are various grades of lung injury by BPA through balloon or wire, and are graded as a transient drop in oxygen saturation, cough, haemoptysis, severe bleeding and adult respiratory distress syndrome. In rare instances, lung injury may occur as reperfusion injury after a delay of 24–48 h after BPA. Other thoracic complications include large pulmonary artery perforations and bleeding. Dissections are oftentimes due to lifting of layers of thrombus by the guiding catheter and contrast injection, and are benign.
Non-thoracic complications are contrast-induced nephropathy, local haematomas at access sites, allergies and side effects of periprocedural medications.
The main complication of BPA is lung injury, which occurred in 9.1% of sessions in the French Registry, in 6.4% of sessions in the Polish Multicenter Registry and in 9.4% of sessions in the Bad Nauheim report.7,30,55 Lesion type did not predict the occurrence of pulmonary injury, but mean pulmonary artery pressure and operator experience predicted complications.7,41
Measures to Improve the Safety of Balloon Pulmonary Angioplasty
Operator experience and BPA strategy appear to be key targets to improve safety.56,57 According to the French group, approximately 100–150 self-responsible procedures safeguard maturity of a BPA interventionist. Technically, the use of hand injection and 50/50 saline-diluted iodixanol 320 are recommended.58 Caution, the use of small balloons or pressure wire-guided angioplasty in case of severe haemodynamic compromise (mPAP >40 mmHg and/or PVR ≥7 Wood units), with the goal of not exceeding an mPAP of 20 mmHg downstream, are mandatory.59
Conclusion
Although the safety of BPA has improved significantly over the years, with a small periprocedural mortality of 0.2% today, questions remain regarding the definition of complete BPA.60 Is the definition of a complete BPA personalised to a patient’s needs? Or is the most appropriate definition of BPA haemodynamic normalisation, restoration of right ventricular function or an mPAP/cardiac output slope of >3 mmHg/l/min under exercise? What is the most reasonable basis for deciding between PEA and BPA with regard to best outcomes?61 More insights, including more data on sex-specific outcomes, are expected from the results of the International BPA Registry.