Atherosclerotic cardiovascular disease (ASCVD) is an important cause of disability and is the leading cause of death globally.1 Targeting modifiable cardiovascular risk factors, such as diabetes, hypercholesterolaemia, hypertension, smoking, physical inactivity and obesity, improves CVD outcomes in individuals with established CVD or those at high risk of CVD. Rheumatoid arthritis (RA) is a chronic inflammatory joint disease (IJD) with a prevalence of 0.5–1%. It is characterised by symmetric polyarthritis and, in the majority of RA patients, formation of autoantibodies, such as rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA). Similar to diabetes, RA is recognised as an independent risk factor for CVD and the key underlying pathology is systemic inflammation.
During the past few decades, mounting evidence on CVD risk in RA has led to substantial advances in CVD risk management in this patient population.2 RA patients are still undertreated in terms of primary and secondary CVD prevention.3,4 Alongside targeting traditional CVD risk factors, suppressing disease activity and inflammation with antirheumatic treatment is expected to reduce CVD burden in these patients. Inflammation plays a major role in atherosclerosis and it has been recognised that among well-treated ASCVD patients without IJD, low-grade inflammation is a key determinant of residual CVD risk.5 Groundbreakingly, anti-inflammatory therapies are being studied for the treatment of ASCVD outside the context of IJDs.
In this review, we summarise the evidence on CVD risk in RA with emphasis on inflammation and the impact of antirheumatic treatment on CVD risk. We also discuss the current knowledge on anti-inflammatory therapies for secondary prevention of ASCVD from a rheumatological point of view.
Epidemiology of CVD in RA
People with RA have an approximate 1.5-fold risk of cardiovascular events and mortality compared to the general population.6,7 This excess cardiovascular burden is comparable to that of people with diabetes, although it is not as well recognised or structurally managed.8
According to a meta-analysis of observational studies, the risk of MI in people with RA compared to the general population is increased by 70% with no differences in the relative risk for men and women.6 The increase in the risk of coronary heart disease (CHD) seems to occur early on in the course of RA, or according to some studies, even before RA is diagnosable.10 Patients with RA are less likely to report symptoms of angina and more likely to experience unrecognised MI and sudden cardiac death than the general population, suggesting an overrepresentation of vulnerable coronary plaques in patients with RA.10 After an MI, RA patients have impaired prognosis compared to the general population in terms of recurrent ischaemia and mortality.11,12
In general, cardiovascular mortality rates have declined during the past 50 years and this has also translated into better survival in RA. Presumably due to marked advances in RA pharmacotherapy and improved control of disease activity, recent studies suggest that the gap in cardiovascular mortality between RA patients diagnosed in the 2000s and the general population may be narrowing or even closing.13,14
Risk of cerebrovascular accidents is increased among RA patients by 40% compared to the general population.6 Alongside ASCVD, RA patients are at increased risk of other types of CVD, such as AF and heart failure.2 Heart failure in RA may partly result from increased prevalence of CHD, but the risk of non-ischaemic heart disease and diastolic dysfunction is also aggravated.15
Traditional Risk Factors do not Fully Explain Excess CVD Risk in RA
RA patients are more likely to suffer from insulin resistance, abnormal fat distribution, be physically inactive and smoke cigarettes compared to non-RA controls.16 Tobacco smoking is the strongest environmental risk factor for RA. A large international RA cohort study demonstrated that about a quarter of cardiovascular events were attributable to smoking (population attributable risk being 37.2% for men and 18.1% for women).17 Diabetes may be more prevalent in RA than in controls, although significant heterogeneity exists between studies.16 Data regarding overrepresentation of hypertension among RA patients is controversial, but most reports indicate no difference.16
Traditional CVD risk factors, however, do not fully explain the observed excess cardiovascular morbidity and mortality in RA. RA-related factors, such as disease activity and burden, extra-articular disease manifestations, elevated inflammatory markers and RF/ACPA positivity attribute to about 30% of the 10-year risk of cardiovascular events.17 A well-powered US-based registry study examined the relationship between longitudinally measured RA disease activity and the risk of CVD and demonstrated that treating RA from high disease activity to remission was associated with an approximate 50% reduction in risk of cardiovascular events.18 Both exposure to each RA flare and cumulative disease activity seem to lead to an increased risk of CVD.19 In some studies, excess cardiovascular mortality risk has been confined only to seropositive RA patients.20
Inflammation Drives CVD Risk in RA and the General Population
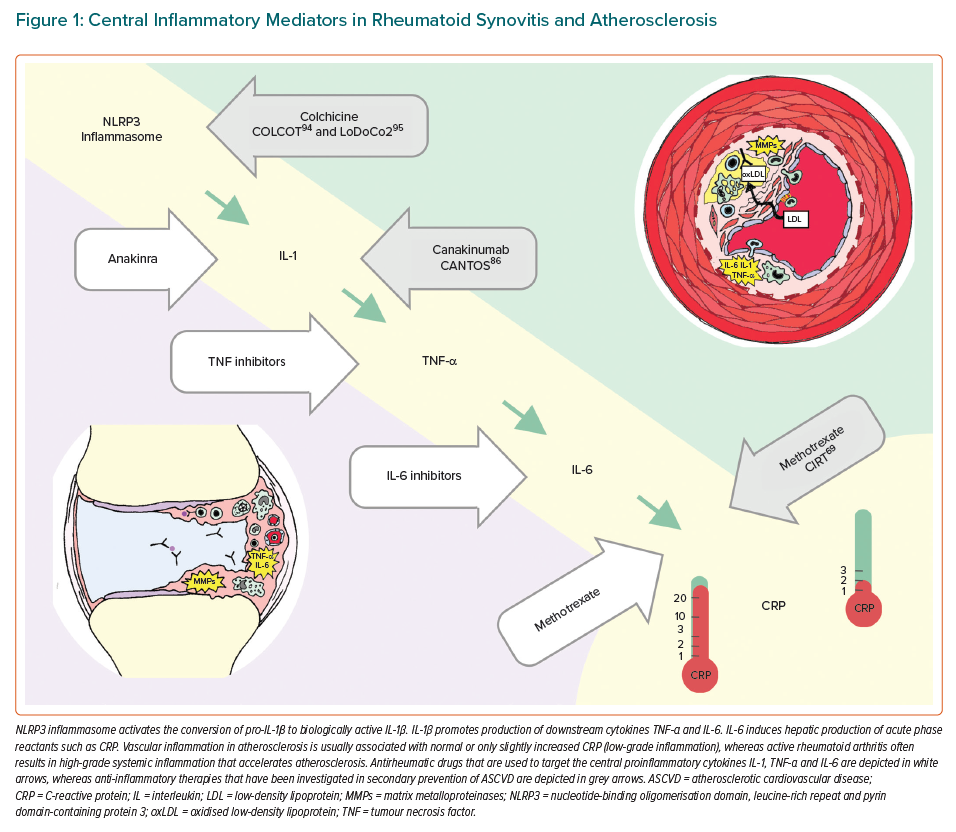
Development of an atherosclerotic plaque is not passive deposition of cholesterol in the vascular wall but an active inflammatory process involving the innate and adaptive immune system. Next, we will highlight the role of selected inflammatory mediators, which are central to pathogenesis of RA, on the development of atherosclerosis, such as nucleotide-binding oligomerisation domain, leucine-rich repeat and pyrin domain-containing protein 3 (NLRP3) inflammasome, and proinflammatory cytokines interleukin 1 (IL-1), tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6). These inflammatory mediators are all interconnected in signalling pathways and they are targets for drugs that are used to treat rheumatic diseases and have gained interest as potential drugs for secondary prevention of ASCVD (Figure 1).
Similar to high-sensitivity C-reactive protein (hsCRP), the role of IL-6 in prediction of cardiovascular events in the general population has been confirmed in primary and secondary prevention cohorts.5 To highlight a few studies, increased levels of IL-6 were associated with the risk of future MI in a prospective study with almost 15,000 apparently healthy men.21 In a secondary analysis of the CIRT study, IL-6 and hsCRP predicted major recurrent cardiovascular events in patients with prior MI or multivessel CHD who also had diabetes or metabolic syndrome, despite modern CVD preventive polypharmacotherapy and high rates of coronary revascularisation.22 IL-6 is also a marker of poor prognosis in acute coronary syndrome (ACS).23
Based on two Mendelian randomisation analyses, IL-6 signalling may have a causal role in the development of ASCVD.24,25 In MI patients, IL-6 production occurs at the site of plaque rupture, as indicated by higher concentration of IL-6 locally compared to levels in systemic circulation.26 IL-6 may also be involved in ischaemia-reperfusion myocardial injury.27 In contrast, CRP is produced in the liver in response to IL-6, and in fact, hsCRP seems to be an easy-to-use downstream marker of IL-6 activation rather than a causal agent in atherogenesis.
NLRP3 inflammasome is a macromolecular protein complex of the innate immune system, which can be activated by many stimuli, such as urate crystals in gout or cholesterol crystals within an atherosclerotic plaque.28 IL-1β and NLRP3 inflammasome are important IL-6 upstream activators. Preclinical evidence links IL-1 to impaired vasodilatation, oxidative stress, plaque formation, growth and rupture.29 IL1-α may contribute to infarct size, whereas IL-1β is associated to adverse cardiac remodelling.29 Based on an ex vivo rodent model of myocardial ischaemia reperfusion, NLRP3 inflammasome may contribute to infarct size.30 Levels of TNF-α have also been demonstrated to predict future cardiovascular events.31 TNF-α is an important regulator of vascular homeostasis and contributes to the development of endothelial dysfunction and a prothrombotic state.32 Among RA patients, increased concentrations of both TNF-α and IL-6 are associated with increased prevalence of coronary artery calcification, independently of Framingham risk score or the presence of diabetes.33
Systemic Inflammation in RA Modifies Traditional CVD Risk Factors
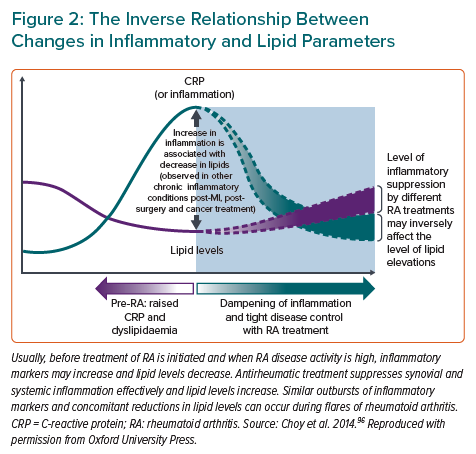
The associations of lipids with CVD risk in RA are complex and essentially linked with inflammation. The levels of total cholesterol (TC), HDL cholesterol (HDL-C) and LDL cholesterol (LDL-C) during active RA and ongoing inflammation typically decrease (Figure 2), a trend that is seen also in septicaemia and other inflammatory states.34 Low lipid levels during inflammation are, however, not associated with lower CVD risk, but rather the opposite.34 This phenomenon is referred to as the lipid paradox.35 In 2011, a retrospective cohort study among 651 RA patients reported a non-linear association of CVD risk and TC levels, with increasing CVD risk at TC levels below 4 mmol/l.35 Recently, this finding was confirmed in a study pooling four of the largest North American RA cohorts with information on coronary arterial calcium (CAC) scores: in contrast to controls, the association of LDL-C with CAC score in RA patients was U-shaped and the largest relative difference in coronary atherosclerosis between the RA and control groups was found among those with an LDL-C lower than 1.8 mmol/l.36 In addition, it was shown that CAC scores were higher in RA patients than in controls across LDL-C levels.36 A possible mechanism for the increased CVD risk despite low LDL-C is that inflammation promotes the oxidation of LDL-C, which is proatherogenic.37 HDL-C particles of RA patients may be unable to prevent LDL oxidation more often than those in controls.38
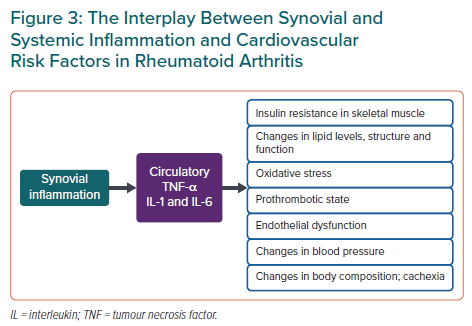
In addition to lipid levels, inflammation modifies other CVD risk factors such as insulin resistance, body composition and blood pressure (Figure 3). A 2020 study exploring the effects of CRP levels on systolic blood pressure in both an RA cohort and a large non-RA outpatient cohort revealed a biphasic association of systolic blood pressure with CRP (with a positive association at CRP <6 mg/l and inverse at CRP >6 mg/l).39 Body mass index (BMI) may also have a paradoxical effect on mortality in RA: patients with the lowest BMI seem to have the highest mortality rates.40 This might be due to the presence of rheumatoid cachexia, sarcopenia and frailty among those with prolonged high disease activity and hence higher CVD risk. Proinflammatory cytokines TNF-α and IL-6 induce insulin resistance, and many antirheumatic agents, including hydroxychloroquine, TNF-α-, IL-1- and IL-6-inhibitors, seem to improve markers of glucose metabolism in RA patients.41
Prevention of CVD in RA
Estimation of Cardiovascular Risk
The accurate estimation of CVD risk in RA and other IJDs is challenging and remains an area of active research. Patients tend to underestimate their CVD risk, warranting more patient education on this topic.42 In 2017, the European League Against Rheumatism (EULAR) published an update on recommendations for CVD risk management in RA and other IJDs.43 These recommendations emphasise that the rheumatologist is responsible for making sure that CVD risk management is addressed in patients with RA and suggest CVD risk assessment for all RA patients at least once every 5 years and reconsidered after major changes in antirheumatic therapy. Lipids should ideally be measured when RA is in remission due to the impact of inflammation on lipoprotein levels.43
CVD risk management among RA patients is performed either by rheumatologists during outpatient visits, GPs collaborating with rheumatologists or, less often, cardiologists cooperating with rheumatologists in a cardio-rheumatology clinic.44 A simple suggestion for CVD risk assessment in rheumatology clinics is that if blood pressure and lipids are recorded along with the already standardised recording of gender, age and smoking status, CVD risk can be calculated based on the Systematic Coronary Risk Evaluation (SCORE) or other CVD risk prediction algorithms. If the recommended treatment threshold is exceeded, patients could be referred to their primary care physician or a cardiologist for CVD prevention measures.45
Most established CVD risk calculators, such as SCORE, and the Reynolds and Framingham risk scores, incorporate only traditional risk factors and underestimate CVD risk among RA patients.46 QRISK2 and QRISK3 include RA as an independent CVD risk factor with a weight of 1.4 and have been shown to inaccurately predict CVD risk in RA.46 The 2018 American College of Cardiology and American Heart Association (ACC/AHA) guidelines on the management of blood cholesterol suggest that RA should be considered as a ‘risk enhancer’, promoting statin therapy in patients with intermediate or borderline risk.47 The 2015/2016 EULAR recommendations state that CVD risk scores should be adapted for patients with RA by a 1.5 multiplication factor if RA is not already included in the algorithm in use.43
Substantial efforts have been made to develop an RA-specific CVD risk prediction model, but with only modest results. An international consortium – A Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis (ATACC-RA) – including 13 RA cohorts from 10 countries developed two risk prediction models that included either a measure of disease activity or functional status but disappointingly, have been found to not improve discrimination when compared with established CVD prediction models.48 A similar well-powered registry study from North America presented a model incorporating measures of disease activity, disability, daily use of prednisolone and disease duration.49 It performed well in internal validation, but failed to improve discrimination compared to established risk calculators in external validation.49,50
Ultrasonography of carotid arteries is a promising non-invasive method to improve CVD risk prediction in RA. It is already incorporated into clinical practice in a few rheumatology clinics. Multiple studies have concluded that RA patients have higher prevalence of asymptomatic carotid atherosclerosis than non-RA controls.51 Carotid ultrasound may markedly improve CVD risk evaluation in RA as was shown in a study of 355 patients with IJDs where 30–60% of patients with an estimated low-to-moderate CVD risk according to established CVD risk calculators had carotid plaques and it was therefore reclassified to very high-risk category with an indication for lipid-lowering therapy.52
Management of Traditional Cardiovascular Risk Factors
Management of traditional CVD risk factors among RA patients is fundamental and includes advice about maintaining a healthy diet, regular exercise, weight control, smoking cessation and treatment with antihypertensive and lipid-lowering agents. Compelling evidence comparing different primary prevention strategies in RA are lacking and risk factor management is recommended to be carried out according to national guidelines.2,43 This means that treatment strategies, thresholds and targets for antihypertensive, anti-diabetic and lipid-lowering therapies are the same in RA and the general population.
It may be asked whether primary prevention of CVD with statins could be beneficial to RA patients irrespective of their estimated CVD risk. A multicentre randomised controlled trial (RCT) TRACE-RA compared the effect of 40 mg daily atorvastatin versus placebo on hard CVD endpoints in RA patients.53 Due to unexpectedly low cardiovascular event rates, the study was terminated prematurely after recruiting only 3,002 RA patients with a median follow-up of 2.5 years. The reduction in cardiovascular events in the atorvastatin versus the placebo arm was estimated to be 34% but was not statistically significant probably due to lack of power caused by short observation period and low number of patients. Although, most importantly, this large risk reduction of 34% has been found in other large statin studies with hard CVD endpoints.54
Statins seem to be equally safe and effective among RA patients and the general population. A post hoc analysis of two large secondary prevention trials demonstrated that the effect of statins on reduction of lipids and cardiovascular event rates among patients with and without IJDs was comparable, despite lower baseline lipid levels in the IJD group.55 Statins may also ameliorate RA disease activity and reduce CRP and erythrocyte sedimentation rate (ESR) levels through their pleiotropic effects.56
Smoking cessation in patients with RA is of utmost importance. An international multicenter study showed that smoking cessation among RA patients is a predictor of reduced rate of cardiovascular events (for former versus current smokers HR 0.70; 95% CI [0.51–0.95]; p=0.02, and for never versus current smokers HR 0.48 ; 95% CI [0.34–0.69]; p<0.001).57 Smoking cessation was also associated with lower disease activity.57 A trial of intensive smoking cessation intervention on RA disease activity (NCT02901886) is currently under way. Every RA patient who smokes should receive advice for smoking cessation and, in our opinion, smoking cessation programmes would ideally be implemented in rheumatology clinics.
Patient education is the key to lifestyle modifications. All patients should receive sufficient information on CVD risk associated with RA in oral and written form. Heart friendly diet recommendations are the same for RA patients as for the general population. Even brief standardised advice on a heart friendly diet may result in a change in nutritional habits, as implied by a small pilot study among IJD patients.58 Barriers to physical activity in RA include pain, fatigue and stiffness, but on the other hand, exercise may aid symptom management, pain relief and joint function.59
Antirheumatic Treatment and Cardiovascular Risk in RA and Beyond
The antirheumatic medications used in the treatment of RA comprise conventional synthetic disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, biological DMARDs such as TNF-α-, IL-6- or IL-1-inhibitors, and targeted synthetic DMARDs, such as Janus kinase (JAK) inhibitors. The pharmacodynamics for conventional synthetic DMARDs is not fully elucidated, whereas biological and targeted synthetic DMARDs affect specific stages of inflammatory signalling.
Suppression of disease activity with antirheumatic treatment is fundamental in lowering CVD risk. Although the mechanisms remain poorly understood, observational studies demonstrate an association of many DMARDs and lowered CVD risk in RA. An increasing number of clinical trials have also shown positive effects of several DMARDs on surrogate markers of CVD among RA patients, but RCTs with hard CVD endpoints are lacking.60–63
In the following sections, we will discuss the cardiovascular effects of DMARDs among RA patients and summarise the published RCTs that explore anti-inflammatory therapies in secondary prevention of ASCVD in patients without IJD. Potential anti-inflammatory therapies for ASCVD, which are not used in the treatment of RA and do not target the central IL-1, TNF-α and IL-6 pathways are not included in this review.
Methotrexate
Methotrexate is the anchor drug of RA treatment and the most important part of the initial antirheumatic treatment strategy. It can be combined with other synthetic or biological DMARDs. Its effects may be mediated at least partly via increased cellular adenosine release.64 Methotrexate may also have direct atheroprotective effects: in a model of cholesterol-fed rabbits, methotrexate reduced the size of atherosclerotic lesions by 75% and also diminished macrophage migration and presence of apoptotic cells in the vessel wall.65
From the beginning of the 2000s, mounting evidence from observational studies among RA cohorts point to an association of methotrexate use and reduced cardiovascular morbidity and mortality. In one prospective study of 1,240 RA patients with a mean follow-up of 6 years, after adjustment for relevant prognostic factors, cardiovascular mortality was reduced by as much as 70% and overall mortality by 60% in patients on methotrexate versus those never treated with methotrexate.66
According to a 2015 meta-analysis, methotrexate use for IJDs was associated with a 28% reduction in fatal and non-fatal cardiovascular events, based on eight observational studies.67 A recently published, well-powered retrospective study using Medicare claims data on RA patients who were initiating biological DMARDs showed that concomitant methotrexate use was associated with a decreased risk of cardiovascular events also among biological DMARD users.68 Unmeasured confounding is naturally a threat to the validity of these findings.
Ridker et al. conducted a large RCT of low-dose methotrexate compared with placebo in 4,786 patients with previous MI or multivessel CHD and either type 2 diabetes or metabolic syndrome (CIRT).69 The study was terminated after a median follow-up of 2.3 years and methotrexate did not affect the rate of a composite CVD endpoint or the levels of IL-1β, IL-6 and CRP. It may be speculated whether an enrichment strategy with hsCRP to guide enrollment of patients into the study would have affected the outcome of the trial (median hsCRP at randomisation was only 1.6 mg/l). In contrast to ASCVD patients with low-grade inflammation, high RA disease activity often results in high-grade inflammation, which in most patients is well-controlled with methotrexate. Thus, it seems logical that the effect of methotrexate on CVD endpoints in RA is different from patients without IJD.
Other Conventional Synthetic DMARDs
Hydroxychloroquine, originally an antimalarial drug, may have cardioprotective effects through favourable changes in lipid and glucose metabolism.70 A safety pilot study of hydroxychloroquine compared with placebo for patients with MI to prevent recurrent cardiovascular events (NCT02648464) has now recruited 125 patients and if no important safety signals arise, the study will most likely be expanded to involve 2,500 patients. In one cross-sectional cohort of 4,363 patients with RA from 15 countries, use of not only methotrexate and biological DMARDs but also use of leflunomide and sulfasalazine was associated with a reduction in the risk of cardiovascular events.71 Leflunomide causes hypertension, and blood pressure must be monitored during treatment.72
TNF-α Inhibitors
If treatment with conventional synthetic DMARDs fails and RA disease activity is high, there is an indication for treatment with biological DMARDs. The most frequently prescribed biological DMARDs are TNF-α inhibitors, which have been in clinical use for two decades. In a 2015 meta-analysis of observational cohort studies among patients with IJD, anti-TNF-α therapy was associated with a 30% reduction in all cardiovascular events, 41% reduction in MIs, 43% reduction in strokes and up to 70% reduction in major adverse cardiac events (MACEs) when compared to other antirheumatic treatments.67 However, the decrease in ACS risk may not be similar in all patients treated with TNF-α inhibitors but conditional to treatment response. In a Swedish registry study among TNF-α inhibitor initiators with RA, 1-year risk of ACS was reduced by 50% among those with good treatment response compared to non-responders.73 In addition, compared with the general population, non-responders had a doubled risk of ACS but those with a good response had equal risk.
TNF-α inhibitors may improve endothelial function and carotid intima-media thickness among RA patients.61,63 However, in contrast to RA patients with high disease activity, patients with low disease activity or remission present no difference in carotid ultrasound findings or arterial stiffness compared with non-RA controls, suggesting that deceleration of these pre-atherosclerotic changes may be related to effective disease control rather than a certain DMARD.74
In the 1990s, preclinical data suggested that TNF-α played a role in the evolution and progression of heart failure. However, RCTs on TNF-α inhibitors etanercept and infliximab for moderate-to-severe heart failure did not show a beneficial effect on hospitalisation or death due to chronic heart failure.75,76 In fact, in the group with the highest infliximab dose of 10 mg/kg, the combined risk of death or hospitalisation for heart failure through 28 weeks compared to placebo was almost tripled.76 As a consequence, New York Heart Association III–IV heart failure is a contraindication for TNF-α inhibitors.
IL-6 Inhibitors
Tocilizumab and sarilumab are anti-IL-6 receptor monoclonal antibodies that are used in the treatment of RA. They are highly effective in suppressing systemic inflammation and normalising the production of acute phase reactants, especially CRP. IL-6 inhibitors increase lipid levels, which has raised concerns about their cardiovascular safety. A Phase IV non-inferiority trial addressed this issue by comparing tocilizumab to etanercept among 3,080 patients with seropositive RA and at least one CVD risk factor. During a mean follow-up of 3.2 years, the occurrence of MACE did not differ between the groups.77
Recently, signs of an atheroprotective effect of IL-6 receptor blockade in RA have been published. A meta-analysis of observational studies suggested that exposure to tocilizumab was associated with a lower risk of MACE but not stroke compared to exposure to TNF-α inhibitors.78 Despite elevations in TC, HDL-C and LDL-C, tocilizumab treatment incurs no change in TC/HDL-C ratio, and it may modify HDL particles towards an anti-inflammatory composition and also reduce lipoprotein (a) levels, which altogether may modify the risk towards a more favourable CVD outcome.60,79 Concomitant statin treatment with tocilizumab seems to be effective for lipid reduction.80 Of note, in patients using IL-6 inhibitors treatment with fluvastatin, pravastatin or rosuvastatin should be chosen, since IL-6 inhibitors may affect cytochrome P450 3A4 (CYP3A4) activity and thus the efficacy of other statins.2
The effect of IL-6 receptor blockade has also been explored for ACS. In an RCT including 121 patients with non-ST-elevation MI (STEMI) scheduled for coronary angiography, a single dose of tocilizumab not only lowered hsCRP levels as expected, but also attenuated the increase in troponin T during hospitalisation, suggesting a smaller infarct size.81 These findings are being further explored in an ongoing study (NCT03004703), in which 200 patients with first-time STEMI will be randomised to receive either tocilizumab or placebo prior to percutaneous coronary intervention with a primary endpoint of myocardial salvage index measured by cardiac MRI 3–7 days after the intervention.
IL-1 inhibitors
There are currently three IL-1 inhibitors available on the market: the IL-1 receptor antagonist anakinra, the soluble decoy receptor rilonacept and the monoclonal IL-1β antibody canakinumab. Of these, only anakinra is approved for the treatment of RA and its efficacy compared to other biological DMARDs is limited. In contrast, IL-1 inhibitors are highly effective in the treatment of certain autoinflammatory diseases. Evidence from rather small clinical trials imply that anakinra may reduce glycated haemoglobin levels among RA patients with diabetes and improve cardiac function, i.e. left ventricular longitudinal strain and coronary flow reserve.82,83
Cardiovascular effects of anakinra have been explored outside rheumatic diseases. In an RCT among 182 non-STEMI patients, 14-day treatment with anakinra attenuated inflammatory response following MI.84 In two pilot studies with a total of 70 patients hospitalised for STEMI, anakinra not only blunted inflammatory response but also improved left ventricle systolic volume index in cardiac MRI and seemed to reduce the incidence of new-onset heart failure compared to placebo, suggesting a favourable effect on left ventricle remodelling and function.85
A pivotal proof-of-concept trial for the inflammatory hypothesis of atherothrombosis, CANTOS, recruited 10,061 patients with prior MI and persistent elevation of hsCRP >2 mg/l. Canakinumab 150 mg, but not 50 mg, reduced the incidence of a combined endpoint of stroke, MI or CVD death by 15% (HR 0.85; 95% CI [0.74–0.98]; p=0.021) compared with placebo over 48 months of follow-up.86 A similar reduction in the primary endpoint was observed for canakinumab 300 mg, but this did not meet the prespecified threshold for statistical significance.
Other Biological DMARDs and JAK Inhibitors
Abatacept, an inhibitor of co-stimulation of T-cells, was found to be associated with a decreased risk of a composite CVD endpoint compared to conventional DMARDs in a large prospective observational study from the US.87 A similar effect was not observed for rituximab, a monoclonal anti-CD20 antibody.
JAK inhibitors are targeted synthetic DMARDs that modulate the cytokine receptor-mediated intracellular signalling cascades. In phase II and III RCTs, a safety signal of a dose-dependent increase in the risk of venous thromboembolism (VTE) was identified with JAK inhibitor tofacitinib.88 However, a recent meta-analysis of RCTs reporting JAK inhibitor safety data revealed no increased risk of all CVD, MACE or VTE, with the exception that baricitinib 2 mg daily was safer than 4 mg with regard to all CVD.89 Caution should be taken when prescribing JAK inhibitors to patients with risk factors for VTE. Similar to IL-6 inhibitors, JAK inhibitors increase cholesterol levels, a side-effect that can be well-controlled with statins.90
Non-steroidal Anti-inflammatory Drugs, Glucocorticoids and Colchicine
Non-steroidal anti-inflammatory drugs (NSAIDs) are often used for rheumatic pain. Long-term use of NSAIDs, especially cyclo-oxygenase-2 selective inhibitors, has been associated with increased risk of CVD. This also holds true in patients with IJD.67 NSAIDs should be used with caution, especially in the presence of CVD risk factors or CVD.43,91
Glucocorticoids have well-known side-effects that amplify CVD risk, including weight gain, hypertension and insulin resistance. Observational studies have demonstrated that low-dose glucocorticoid use in IJD is associated with an approximate 50% increase in CVD risk.67 This association is dependent on glucocorticoid dosage and cumulative exposure.92 It is unclear, however, to which extent these results are affected by confounding by indication. This question may be answered by the GLORIA study which is assessing the harm, benefit and costs of 5 mg prednisolone daily versus placebo added to standard care in RA patients >65 years (NCT02585258). Glucocorticoids should be used for RA with the minimum effective dosage and tapered in case of remission or low disease activity.
Although not used in the treatment of RA but in gout, colchicine, an anti-inflammatory drug that inhibits tubulin polymerisation, microtubule generation and putatively also NLRP3 inflammasome activation, deserves attention (Figure 1).93 Two large RCTs have shown that colchicine reduces the risk of cardiovascular events compared to placebo after acute MI and in chronic CHD.94,95
Conclusion
RA patients have an increased ASCVD burden that is linked to disease activity, systemic inflammation, traditional CVD risk factors and a paradoxical decrease in lipid levels. Rheumatoid synovitis and unstable atherosclerotic plaque share common inflammatory pathophysiological mechanisms, such as expression of proinflammatory cytokines IL-1, TNF-α and IL-6. Increased CVD risk in RA may be reduced by addressing CVD risk factors and suppression of inflammation with effective antirheumatic therapy. However, awareness of this phenomenon is still low in RA patients and healthcare professionals and CVD risk factors often remain undertreated in this high-risk population.
Several DMARDs, especially methotrexate, TNF-α inhibitors and IL-6 inhibitors, seem to improve various biomarkers of CVD and reduce cardiovascular events in observational studies among RA cohorts. It is uncertain, however, whether these observations are due to reduced disease activity in general or to specific atheroprotective effects. The former theory is supported by the notion that CVD risk and its surrogate markers seem to correlate with treatment response to antirheumatic medication. More research is warranted on tailoring of DMARD treatment to RA patients with regard to their individual CVD risk. Furthermore, RA patients would most probably benefit from more structured CVD prevention programmes, such as in diabetes care.
Anti-inflammatory therapies – especially IL-1 inhibitors and colchicine – are promising agents to reduce inflammation-mediated residual risk of recurrent cardiovascular events among ASCVD patients. Further evaluation of IL-6 and IL-1 inhibitors as adjunct therapy for MI is warranted. Lessons from RA, a natural model of high-grade inflammation, can be used to understand the inflammatory mechanisms of atherosclerosis also in the general population and to identify novel targets for ASCVD treatment.